Which Of The Following May Form Linear Polymers
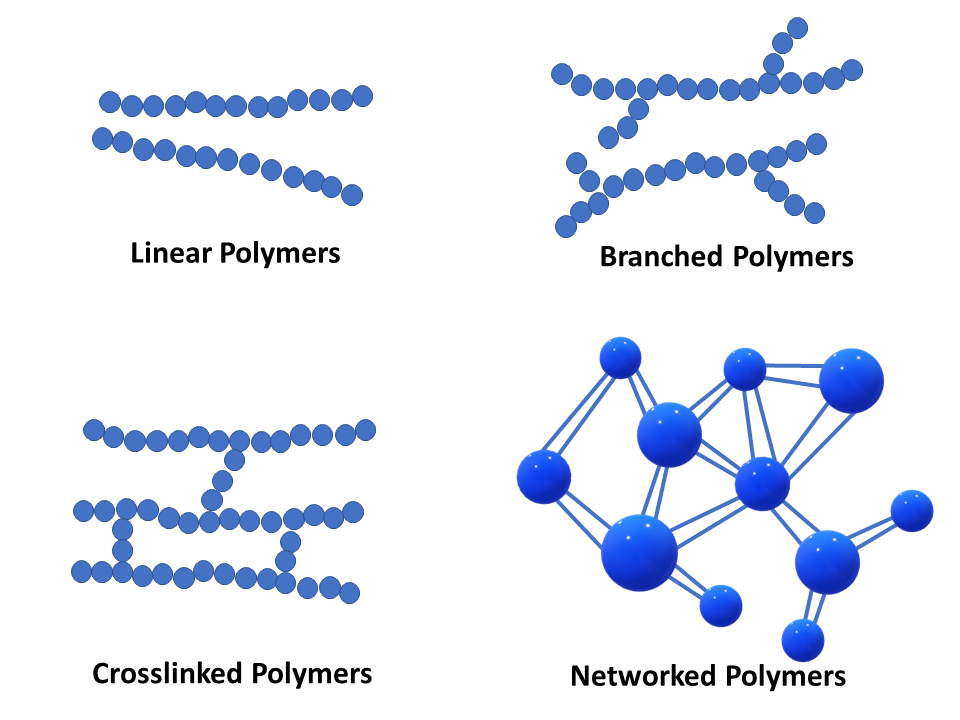
Which Of The Following May Form Linear Polymers - The monomers in these are linked together to form a long chain. Web depending on the structure of the monomer and on the polymerization method employed, polymer chains may show different architectures. Note that for commercial synthesis the carboxylic acid components may actually be employed in the form of derivatives such as simple esters. Web various polymer structures can be produced depending on the monomers and reaction conditions: Web which type (s) of bond (s) is (are) found between atoms within hydrocarbon molecules? Cellulose and natural rubber are an example of. These polymers are similar in structure to a long straight chain which identical links connected to each other. A linear polymer may be considered to be a collection of linear chainlike molecules, such as shown in figure 1.40. Also, the polymerization reactions for nylon 6 and. Linear, branched, and network polymers.a polymer with a simple linear structure is formed.
A regular and symmetrical linear chain, a low degree of polymerization, strong intermolecular forces, small and regular pendant groups, a slow rate of cooling, and oriented molecules. These polymers are composed of the main chain with more than one side chain and thus form branches. In some polymers shorter chains grow off the long chain at certain intervals, so that a branched structure is formed. Web various polymer structures can be produced depending on the monomers and reaction conditions: These polymers are similar in structure to a long straight chain which identical links connected to each other. To account for the physical differences between the different types of polymers, the nature of the aggregate macromolecular structure, or morphology, of each substance must be considered.because polymer molecules are so. These polymers possess a single linear chain with zero branches. Polyvinyl chloride, polystyrene and polyethylene are examples of the polyvinyl family. A polymer may consist of linear macromolecules containing each only one unbranched chain. Web solution verified by toppr correct option is c) cellulose is a natural linear polymer, a long chain made by the linking of smaller glucose molecules.
A polymer may consist of linear macromolecules containing each only one unbranched chain. Web a linear polymer characterized by a repetition of ester groups along the backbone chain is called a polyester. Web solution verified by toppr correct option is c) cellulose is a natural linear polymer, a long chain made by the linking of smaller glucose molecules. Linear, branched, and network polymers.a polymer with a simple linear structure is formed. Starches, an important source of food energy. These polymers are composed of the main chain with more than one side chain and thus form branches. Web the following examples of condensation polymers are illustrative. These polymers possess a single linear chain with zero branches. A regular and symmetrical linear chain, a low degree of polymerization, strong intermolecular forces, small and regular pendant groups, a slow rate of cooling, and oriented molecules. These polymers have high melting points and are of higher density.
Polymer Chemistry 5 Types of Classification of Polymers
The monomers in these are linked together to form a long chain. Web a linear polymer characterized by a repetition of ester groups along the backbone chain is called a polyester. Polyvinyl chloride, polystyrene and polyethylene are examples of the polyvinyl family. These polymers are similar in structure to a long straight chain which identical links connected to each other..
DrakeOwFernandez
Web solution verified by toppr correct option is c) cellulose is a natural linear polymer, a long chain made by the linking of smaller glucose molecules. Polyvinyl chloride, polystyrene and polyethylene are examples of the polyvinyl family. How do the densities of crystalline and amorphous polymers of the same material that have identical molecular weights. Starches, an important source of.
Stimuliresponsive polymer materials showing a variety of topological
Which of the following form network polymers? Web a linear polymer characterized by a repetition of ester groups along the backbone chain is called a polyester. Starches, an important source of food energy. The monomers of polyvinyls have monomers as shown below. Polyvinyl chloride, polystyrene and polyethylene are examples of the polyvinyl family.
Solved Which of the following may form linear polymers?
How do the densities of crystalline and amorphous polymers of the same material that have identical molecular weights. These polymers are composed of the main chain with more than one side chain and thus form branches. The monomers in these are linked together to form a long chain. If the segments are connected through the carbon atoms, then a linear.
Which of the following is a linear polymer ? YouTube
If the segments are connected through the carbon atoms, then a linear polymer chain results. Polyvinyl chloride, polystyrene and polyethylene are examples of the polyvinyl family. Starches, an important source of food energy. The monomers in these are linked together to form a long chain. Web a linear polymer characterized by a repetition of ester groups along the backbone chain.
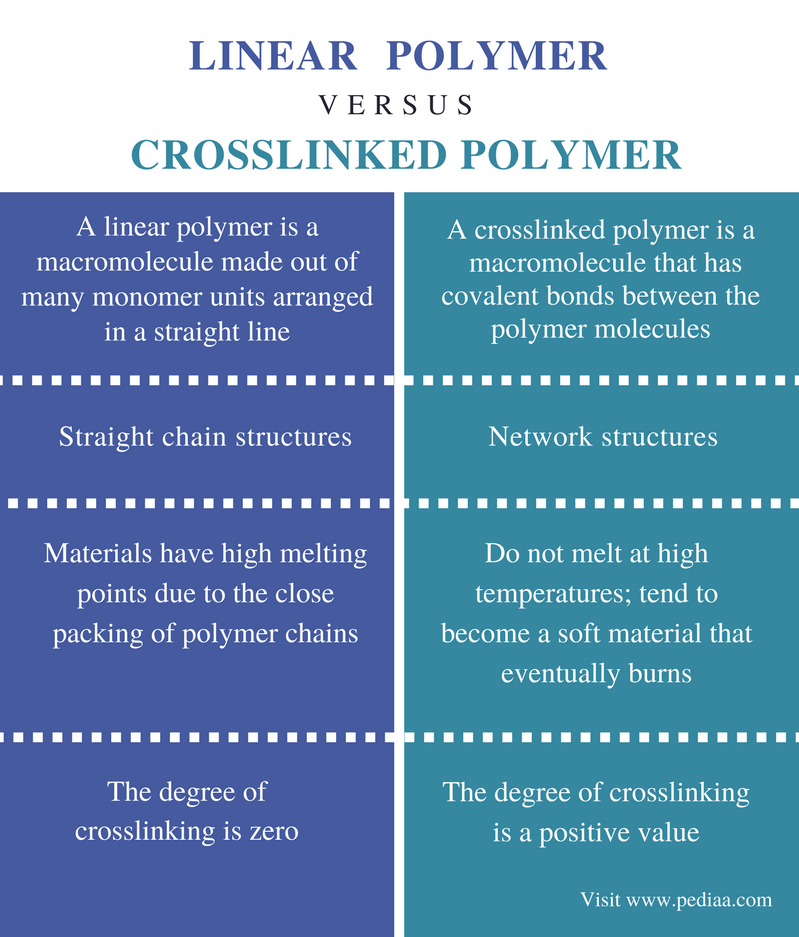
Difference Between Linear and Crosslinked Polymer Definition
Polyvinyl chloride, polystyrene and polyethylene are examples of the polyvinyl family. Web which type (s) of bond (s) is (are) found between atoms within hydrocarbon molecules? A polymer may consist of linear macromolecules containing each only one unbranched chain. These polymers are similar in structure to a long straight chain which identical links connected to each other. Which of the.
PPT Which of the following compounds may be polymers? PowerPoint
Web various polymer structures can be produced depending on the monomers and reaction conditions: Long chains of 10,000 or more monomers can be packed closely to form a. If the segments are connected through the carbon atoms, then a linear polymer chain results. Which of the following may form linear polymers? To account for the physical differences between the different.
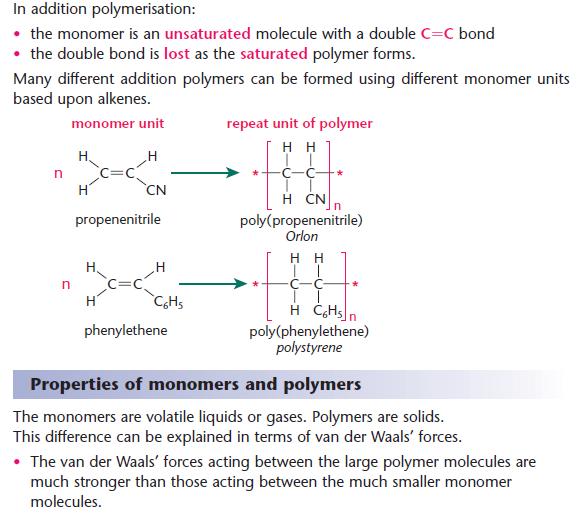
Polymers Chemistry ALevel Revision
A linear polymer may be considered to be a collection of linear chainlike molecules, such as shown in figure 1.40. Cellulose and natural rubber are an example of. These polymers are composed of the main chain with more than one side chain and thus form branches. Web polyvinyls and polyesters are commercial polymers with such linear backbones. Web depending on.
Linear, Branched and Cross Linked Polymers and Polymer Crystallinity
These polymers are similar in structure to a long straight chain which identical links connected to each other. Cellulose and natural rubber are an example of. In some polymers shorter chains grow off the long chain at certain intervals, so that a branched structure is formed. Web six factors favor a polymer with a high percent crystallinity: Web solution verified.
(PDF) Latest Advances on the Synthesis of Linear ABCType Triblock
These polymers are similar in structure to a long straight chain which identical links connected to each other. A linear polymer may be considered to be a collection of linear chainlike molecules, such as shown in figure 1.40. Those with high molecular weights (10,000 to 15,000 molecules) are employed in the. Web depending on the structure of the monomer and.
To Account For The Physical Differences Between The Different Types Of Polymers, The Nature Of The Aggregate Macromolecular Structure, Or Morphology, Of Each Substance Must Be Considered.because Polymer Molecules Are So.
A regular and symmetrical linear chain, a low degree of polymerization, strong intermolecular forces, small and regular pendant groups, a slow rate of cooling, and oriented molecules. Web solution verified by toppr correct option is c) cellulose is a natural linear polymer, a long chain made by the linking of smaller glucose molecules. These polymers possess a single linear chain with zero branches. The monomers in these are linked together to form a long chain.
Web Depending On The Structure Of The Monomer And On The Polymerization Method Employed, Polymer Chains May Show Different Architectures.
These polymers are composed of the main chain with more than one side chain and thus form branches. Web a linear polymer characterized by a repetition of ester groups along the backbone chain is called a polyester. In a solid plastic, a collection of these linear chains may be arranged in a completely random manner—looped, coiled, and. Long chains of 10,000 or more monomers can be packed closely to form a.
Web Polyvinyls And Polyesters Are Commercial Polymers With Such Linear Backbones.
Web which type (s) of bond (s) is (are) found between atoms within hydrocarbon molecules? A linear polymer may be considered to be a collection of linear chainlike molecules, such as shown in figure 1.40. These polymers have high melting points and are of higher density. The monomers of polyvinyls have monomers as shown below.
Note That For Commercial Synthesis The Carboxylic Acid Components May Actually Be Employed In The Form Of Derivatives Such As Simple Esters.
Web the following examples of condensation polymers are illustrative. Starches, an important source of food energy. If the segments are connected through the carbon atoms, then a linear polymer chain results. Web various polymer structures can be produced depending on the monomers and reaction conditions: