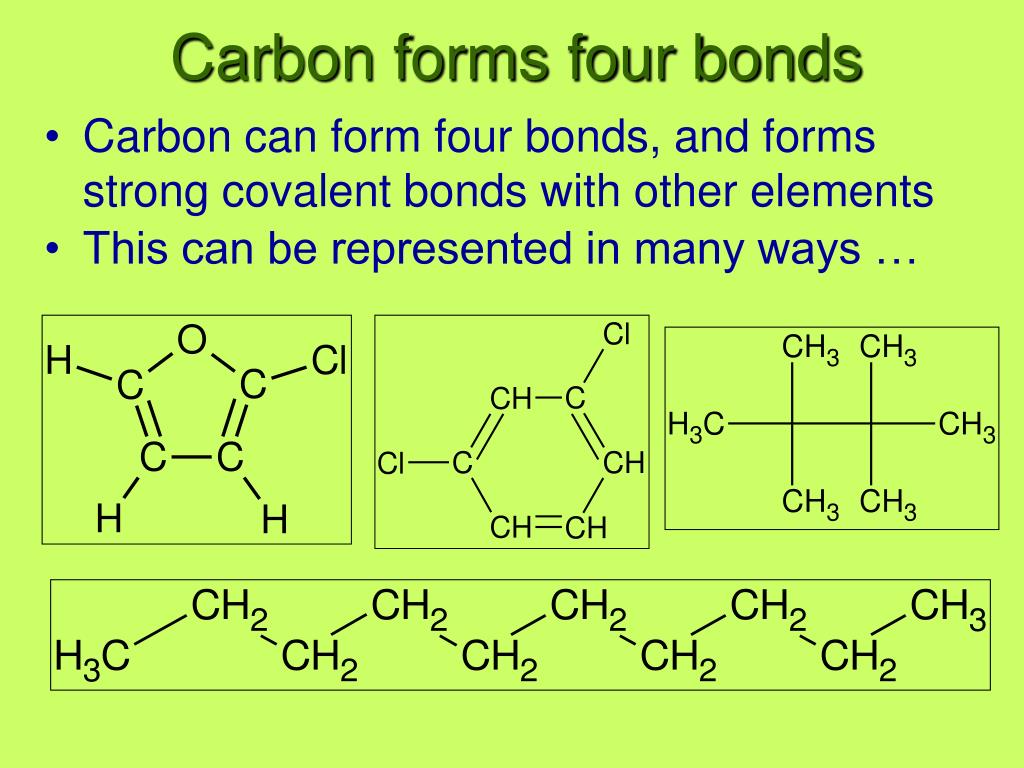
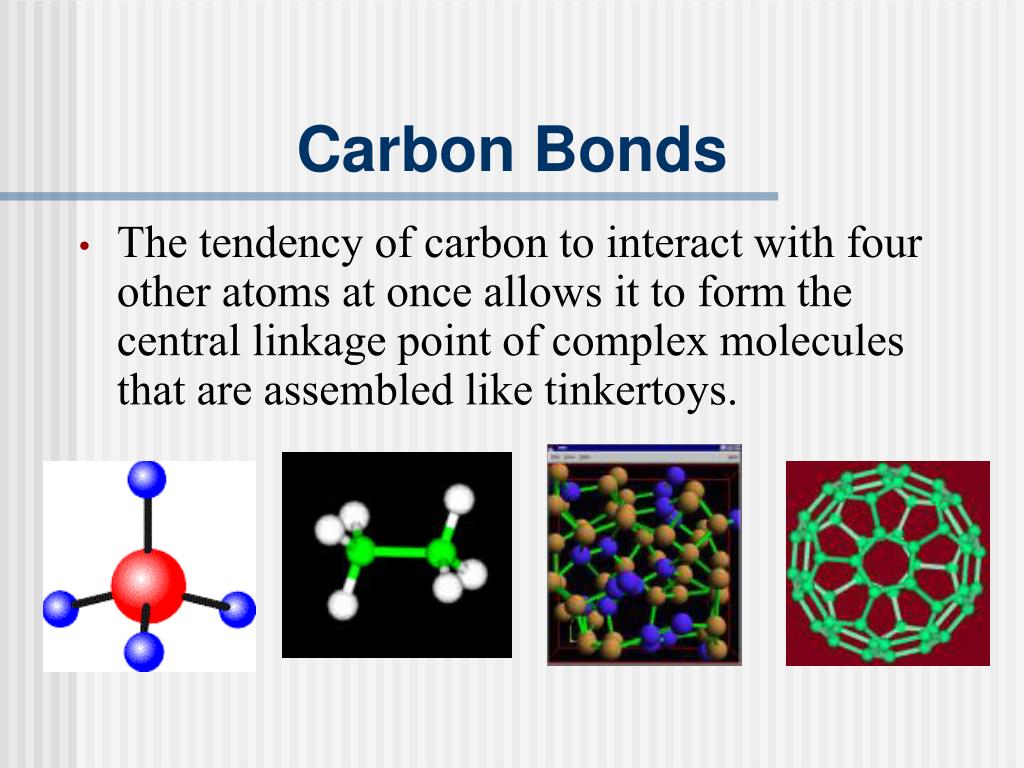
4 Types Of Bonds Carbon Can Form
4 Types Of Bonds Carbon Can Form - Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. Web the most common bond carbon can form is a covalent bond. Web name the 4 types of bonds carbon can form? Single covalent bond is formed when carbon shares one electron pair with another atom. The simplest carbon molecule is methane (ch 4 ), depicted here. In the 1970s, scientists made an. Carbon can form stable bonds with many elements, including itself. A triple bond results when two atoms share three electron pairs to form three. There are four major types of organic compounds:. Web first type of bond that carbon can form is a single covalent bond.
A triple bond results when two atoms share three electron pairs to form three. Web for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Carbon shares electrons with other atoms. Web the four types of bonds that carbon can form are as follows: There are four major types of organic compounds:. Web carbon can form four covalent bonds to create an organic molecule. But that rule doesn’t always hold. This is the most often. Web answer (1 of 9): Web the most common bond carbon can form is a covalent bond.
Web name the 4 types of bonds carbon can form? Carbon can form stable bonds with many elements, including itself. Web carbon can form four covalent bonds to create an organic molecule. Web carbon can form single bonds (sharing of 2 electrons), double bonds (sharing of 4 electrons), and/or a triple bond (sharing of 6 electrons). Carbon shares electrons with other atoms. Single covalent bond is formed when carbon shares one electron pair with another atom. Web carbon is the main element in organic compounds. Web carbon has four such sharable electrons of its own, so it tends to form four bonds to other atoms. Web a double bond is formed when two atoms use two electron pairs to form two covalent bonds; This is the most often.
Quiz & Worksheet What Types of Bonds Can Carbon Form?
Carbon can form stable bonds with many elements, including itself. Carbon shares electrons with other atoms. There are four major types of organic compounds:. Web carbon has four such sharable electrons of its own, so it tends to form four bonds to other atoms. The simplest carbon molecule is methane (ch 4 ), depicted here.
The 4 Types of Bonds Carbon Can Form Video & Lesson Transcript
To form ionic bonds, carbon molecules must either gain or. Single covalent bond is formed when carbon shares one electron pair with another atom. Web the four types of bonds that carbon can form are as follows: There are four types of carbon bonds. Web first type of bond that carbon can form is a single covalent bond.
PPT Organic Chemistry PowerPoint Presentation, free download ID5887001
Web carbon can form single bonds (sharing of 2 electrons), double bonds (sharing of 4 electrons), and/or a triple bond (sharing of 6 electrons). Web carbon is the main element in organic compounds. Carbon shares electrons with other atoms. Web carbon has four such sharable electrons of its own, so it tends to form four bonds to other atoms. A.
Answered 7.10. Carbon can form bonds with itself… bartleby
This is the most often. Web carbon is the main element in organic compounds. Web a double bond is formed when two atoms use two electron pairs to form two covalent bonds; Carbon shares electrons with other atoms. A triple bond results when two atoms share three electron pairs to form three.
PPT Biochemistry PowerPoint Presentation, free download ID89333
Single covalent bond is formed when carbon shares one electron pair with another atom. There are four types of carbon bonds. This is the most often. Web the most common bond carbon can form is a covalent bond. But that rule doesn’t always hold.
How Many Single Bonds Can Carbon Form fredhughesdesign
Web carbon can form four covalent bonds to create an organic molecule. Web a double bond is formed when two atoms use two electron pairs to form two covalent bonds; Web the most common bond carbon can form is a covalent bond. Web in most stable compounds of carbon (and nearly all stable organic compounds), carbon obeys the octet rule.
Chemical Bonds Definition, Types, and Examples
The simplest carbon molecule is methane (ch 4 ), depicted here. Web answer (1 of 9): A triple bond results when two atoms share three electron pairs to form three. Web first type of bond that carbon can form is a single covalent bond. Web carbon can form four covalent bonds to create an organic molecule.
PPT CHAPTER 25 CARBON AND ITS COMPOUNDS PowerPoint Presentation
Web name the 4 types of bonds carbon can form? To form ionic bonds, carbon molecules must either gain or. The simplest carbon molecule is methane (ch 4 ), depicted here. Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. Web first type of bond that carbon can form is a single covalent.
PPT Biochemistry PowerPoint Presentation, free download ID89333
The simplest carbon molecule is methane (ch 4 ), depicted here. Single covalent bond is formed when carbon shares one electron pair with another atom. Web in most stable compounds of carbon (and nearly all stable organic compounds), carbon obeys the octet rule and is tetravalent, meaning that a carbon atom forms a total of four. There are four types.
duermen los delfines en la citacion? MNC COURIER
Web carbon can form single bonds (sharing of 2 electrons), double bonds (sharing of 4 electrons), and/or a triple bond (sharing of 6 electrons). Web the most common bond carbon can form is a covalent bond. Web first type of bond that carbon can form is a single covalent bond. There are four major types of organic compounds:. There are.
Web Carbon Can Form Four Covalent Bonds To Create An Organic Molecule.
Web carbon can form single bonds (sharing of 2 electrons), double bonds (sharing of 4 electrons), and/or a triple bond (sharing of 6 electrons). Web first type of bond that carbon can form is a single covalent bond. In the 1970s, scientists made an. This is the most often.
Web A Double Bond Is Formed When Two Atoms Use Two Electron Pairs To Form Two Covalent Bonds;
Sp^3 is where the four bonds possible with a carbon atom are hybridized from one s orbital and 3 p orbitals into four. But that rule doesn’t always hold. Web in most stable compounds of carbon (and nearly all stable organic compounds), carbon obeys the octet rule and is tetravalent, meaning that a carbon atom forms a total of four. Web the most common bond carbon can form is a covalent bond.
Web Carbon Has Four Such Sharable Electrons Of Its Own, So It Tends To Form Four Bonds To Other Atoms.
Carbon can form stable bonds with many elements, including itself. Web name the 4 types of bonds carbon can form? There are four types of carbon bonds. Web the four types of bonds that carbon can form are as follows:
There Are Four Major Types Of Organic Compounds:.
Web carbon is the main element in organic compounds. A triple bond results when two atoms share three electron pairs to form three. Web answer (1 of 9): To form ionic bonds, carbon molecules must either gain or.