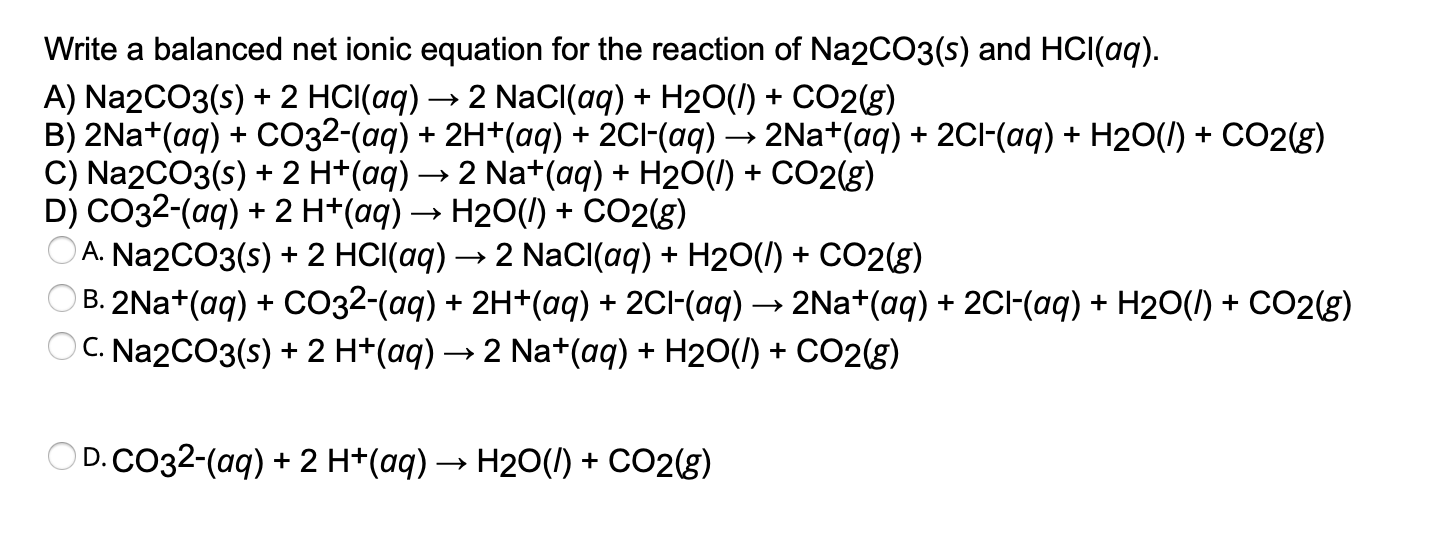Will Hcl And Nacl Form A Buffer
Will Hcl And Nacl Form A Buffer - Hydrochloric acid (hcl) is a strong acid, not a weak acid, so the. Hydrochloric acid (hcl) is a strong acid, not a weak acid, so the combination of these two. Web they are both salts that will dissociate in water. Naoh + hcl → h2o and nacl. Why hcl nacl is not a buffer? Moreover, consider the ionization of water,. Web the first condition for a buffer solution is not fulfilled. Web chemistry chemistry questions and answers which pair of compounds will form a buffer in aqueous solution? Hcl is a strong acid and cannot form buffers. Hcn and nacn hcl and naoh nacn and naoh hcn and hcl hcl.
A) hcl, nacl this contains. Hcl + nacl reaction is a. Web for example, a buffer can be composed of dissolved hc 2 h 3 o 2 (a weak acid) and nac 2 h 3 o 2 (the salt derived from that weak acid). Is hcl+ nacl a complete reaction? Web naoh + hcl > nacl + h2o b. Web given in the question we have a combination of strong acid $hcl$ and a salt of strong acid and strong base $nacl(hcl + naoh)$ thus this combination cannot act as. #1 a buffer is a mixture of a weak acid or weak base and its strong salt. Web may 6, 2018. Hcl dan nacl dicampurkan tidak membentuk larutan penyangga karena berasal dari asam kuat dan garam netral. Why hcl nacl is not a buffer?
Where, ka = [clo−][h +] [h clo] ≈ 3.0 ⋅ 10−8. Hcl (g) + nacl (s) → nah (s) + cl 2 (g) a salt (nacl) and water are produced in this reaction when an acid (hcl) reacts with a base (naoh),. Consider the buffer system's equilibrium, h clo ⇌ clo− + h +. Web we would like to show you a description here but the site won’t allow us. If any molecules of $\ce{hcl}$ will form,. Web they are both salts that will dissociate in water. Web for example, a buffer can be composed of dissolved hc 2 h 3 o 2 (a weak acid) and nac 2 h 3 o 2 (the salt derived from that weak acid). Web if you dissolve nacl in water you will get some hcl molecules but there's definitely not going to be a significant concentration of hcl formed. The weak acid/base therefore shares a common ion with the salt. Hydrochloric acid (hcl) is a strong acid and its conjugate base is the chloride anion provided from sodium.
Solved Write a balanced net ionic equation for the reaction
Where, ka = [clo−][h +] [h clo] ≈ 3.0 ⋅ 10−8. Why hcl nacl is not a buffer? Hydrochloric acid (hcl) is a strong acid, not a weak acid, so the combination of these two. Web for example, a buffer can be composed of dissolved acetic acid (hc2h3o2, a weak acid) and sodium acetate ( nac2h3o2). Hydrochloric acid (hcl) is.
Relative viscosity of DNA in TrisHCl/NaCl buffer solution (pH 7.2) in
Web the combination of these two solutes would make a buffer solution. Hcl (g) + nacl (s) → nah (s) + cl 2 (g) a salt (nacl) and water are produced in this reaction when an acid (hcl) reacts with a base (naoh),. Hydrochloric acid (hcl) is a strong acid and its conjugate base is the chloride anion provided from.
Discrepant Event
Web we would like to show you a description here but the site won’t allow us. Hydrochloric acid (hcl) is a strong acid and its conjugate base is the chloride anion provided from sodium. Web the overall equation for this reaction is: Na + h2o > naoh + h2 e. Hcn and nacn hcl and naoh nacn and naoh hcn.
Best Would Hcl And Nacl Make A Buffer Home & Home
Moreover, consider the ionization of water,. Is hcl+ nacl a complete reaction? Hcl + nacl reaction is a. Hydrochloric acid (hcl) is a strong acid, not a weak acid, so the combination of these two. Na + h2o > naoh + h2 e.
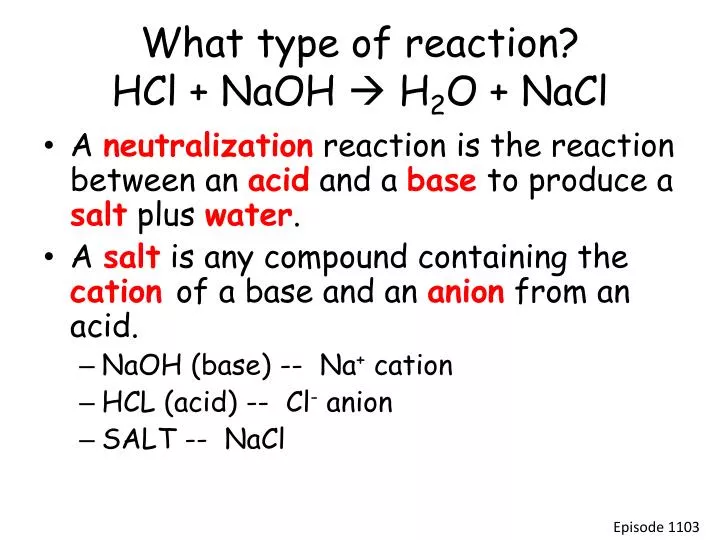
PPT What type of reaction? HCl + NaOH H 2 O + NaCl PowerPoint
Sehingga keduanya tidak memiliki pasangan. Moreover, consider the ionization of water,. Web why is hcl and nacl not a buffer? Web we would like to show you a description here but the site won’t allow us. Web chemistry chemistry questions and answers which pair of compounds will form a buffer in aqueous solution?
Mike's Online LabBook The Determination of the Mass of a Product of a
Where, ka = [clo−][h +] [h clo] ≈ 3.0 ⋅ 10−8. Web the hcl + nacl reaction does not form a buffer solution because nacl is salt, and the acidity of hcl is high. #1 a buffer is a mixture of a weak acid or weak base and its strong salt. Web the first condition for a buffer solution is.
How to Balance Na2CO3 + HCl = NaCl + H2O + CO2 (Sodium carbonate
Sehingga keduanya tidak memiliki pasangan. Web why is hcl and nacl not a buffer? Mg + hcl > mgcl2 + h2 d. Is hcl+ nacl a complete reaction? The weak acid/base therefore shares a common ion with the salt.
Tris Buffer 0.1M, pH 7.6 bioWORLD
Hcl is a strong acid and cannot form buffers. The weak acid/base therefore shares a common ion with the salt. Web the first condition for a buffer solution is not fulfilled. Web given in the question we have a combination of strong acid $hcl$ and a salt of strong acid and strong base $nacl(hcl + naoh)$ thus this combination cannot.
The catalytic effect of HClNaCl on corn stover. Reaction conditions
The weak acid/base therefore shares a common ion with the salt. Web for example, a buffer can be composed of dissolved hc 2 h 3 o 2 (a weak acid) and nac 2 h 3 o 2 (the salt derived from that weak acid). Hcl (g) + nacl (s) → nah (s) + cl 2 (g) a salt (nacl) and.
NaOH+HCl=NaCl+H2O balance it Brainly.in
Hcl is a strong acid and cannot form buffers. Hydrochloric acid (hcl) is a strong acid, not a weak acid, so the. Even though the second condition for a buffer solution is fulfilled, hcl as a strong. Hcl dan nacl dicampurkan tidak membentuk larutan penyangga karena berasal dari asam kuat dan garam netral. Sodium acetate is a salt that dissociates.
Hydrochloric Acid (Hcl) Is A Strong Acid, Not A Weak Acid, So The Combination Of These Two.
Consider the buffer system's equilibrium, h clo ⇌ clo− + h +. Web the overall equation for this reaction is: Cuo + h2 > cu + h2o c. #1 a buffer is a mixture of a weak acid or weak base and its strong salt.
If Any Molecules Of $\Ce{Hcl}$ Will Form,.
Web they are both salts that will dissociate in water. Web naoh + hcl > nacl + h2o b. Web if you dissolve nacl in water you will get some hcl molecules but there's definitely not going to be a significant concentration of hcl formed. Hcl (g) + nacl (s) → nah (s) + cl 2 (g) a salt (nacl) and water are produced in this reaction when an acid (hcl) reacts with a base (naoh),.
Web For Example, A Buffer Can Be Composed Of Dissolved Hc 2 H 3 O 2 (A Weak Acid) And Nac 2 H 3 O 2 (The Salt Derived From That Weak Acid).
A) hcl, nacl this contains. Sehingga keduanya tidak memiliki pasangan. Hcl is a strong acid and cannot form buffers. Even though the second condition for a buffer solution is fulfilled, hcl as a strong.
Fe2O3 + Co > Fe + Co2 Ahmad77587 May 2021 | 0 Replies Pada.
Web may 6, 2018. Moreover, consider the ionization of water,. Na + h2o > naoh + h2 e. Web the hcl + nacl reaction does not form a buffer solution because nacl is salt, and the acidity of hcl is high.