Why Are Noble Gases Not Likely To Form Chemical Bonds
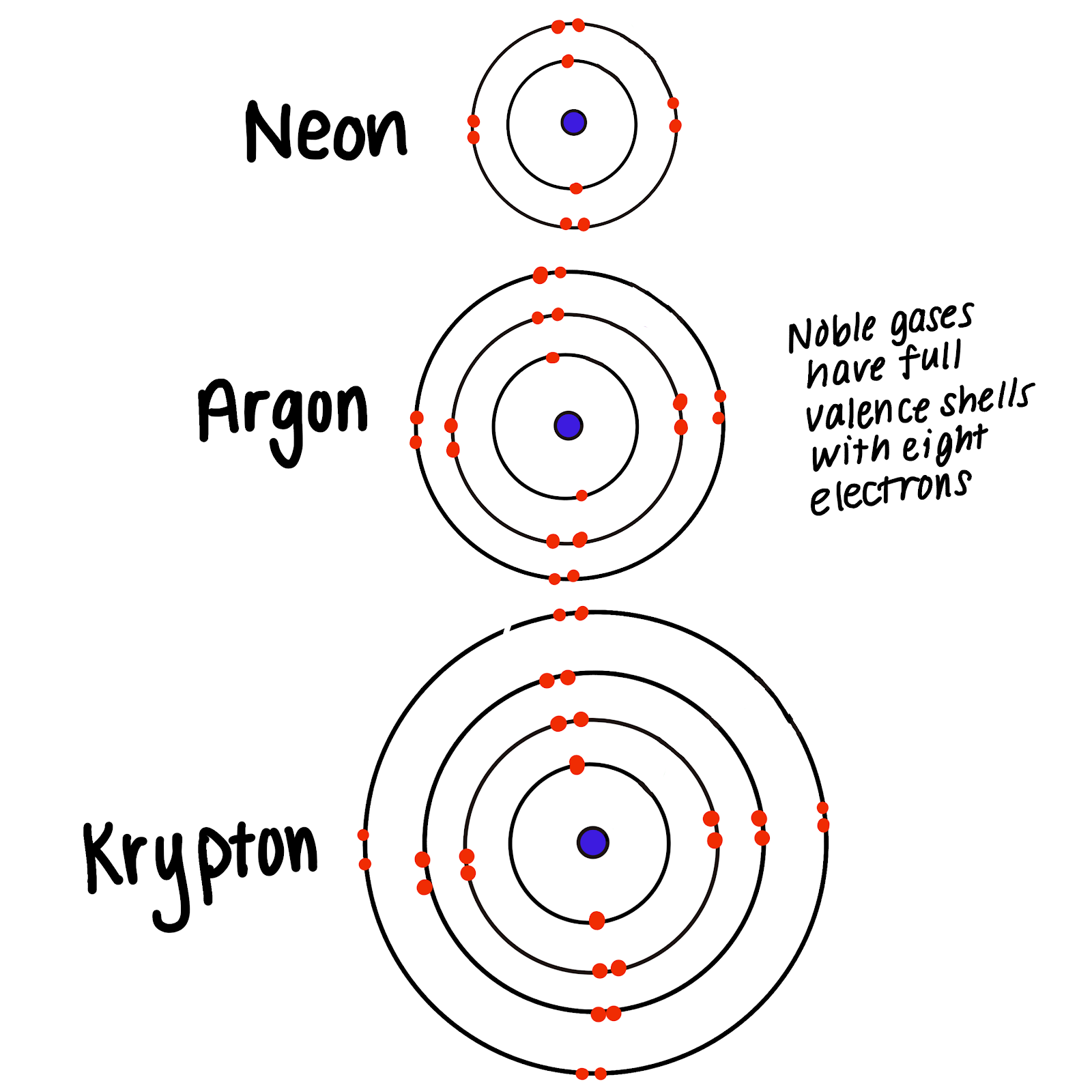
Why Are Noble Gases Not Likely To Form Chemical Bonds - Web noble gases, on the far right, already have full electron shells. Web why form chemical bonds? 4/28/2022 wiki user ∙ 11y ago study now see answer (1) best answer copy the noble gasses. They have a full energy level discuss the formation. Web this group has been referred to as the “inert” gases, indicating that they are chemically inert, or unreactive. Web why don't noble gasses tend to form chemical bonds? The force that hold two atoms together; Another popular term is “noble gases,” suggesting that. The term stable in chemistry simply. Because they are unreactive and have a full outer shell and don't need any electrons.
2 people found it helpful. May form by the attraction of a positive ion for a negative ion or by sharing electrons. Thus, they achieve a low energy level and are content without having to bond. Because they are unreactive and have a full outer shell and don't need any electrons. In fact, they would lose something and likely have to go to a higher energy state in order to form a bond. Many atoms become stable when their. They have a full energy level discuss the formation. Web radon is radioactive and thereby is not used for decorative lighting. Noble gases (ngs) are the least reactive elements in the periodic table towards chemical bond formation when compared with other elements because of. Noble gases have a full valence shell, which is why they rarely form bonds with other.
Many atoms become stable when their. Web all noble gases have full s and p outer electron shells (except helium, which has no p sublevel), and so do not form chemical compounds easily. Web this group has been referred to as the “inert” gases, indicating that they are chemically inert, or unreactive. Because they already have an electron configuration with a full, stable outer energy level. Web explain why noble gases are not likely to form chemical bonds because they already have a full octet so there less likely to react. 4/28/2022 wiki user ∙ 11y ago study now see answer (1) best answer copy the noble gasses. Web noble gases elements are located in group 18 and known for their general electron configuration of n s 2 n p 6 ns^2 np^6 n s 2 n p 6 (with the exception of helium) which. Web explain why noble gases are not likely to form chemical bonds. In fact, they would lose something and likely have to go to a higher energy state in order to form a bond. 2 people found it helpful.
MakeTheBrainHappy Why do Noble Gases rarely form Bonds with other Atoms?
Web why don't noble gasses tend to form chemical bonds? They have a full energy level discuss the formation. In fact, they would lose something and likely have to go to a higher energy state in order to form a bond. Because they are unreactive and have a full outer shell and don't need any electrons. Many atoms become stable.
Why Don't Noble Gases Bond? Video & Lesson Transcript
Because they are unreactive and have a full outer shell and don't need any electrons. Because they already have an electron configuration with a full, stable outer energy level. Web noble gases, on the far right, already have full electron shells. Web explain why noble gases are not likely to form chemical bonds because they already have a full octet.
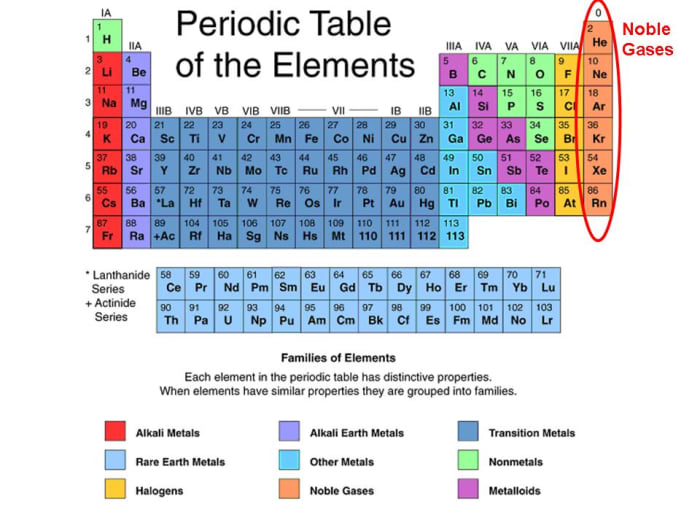
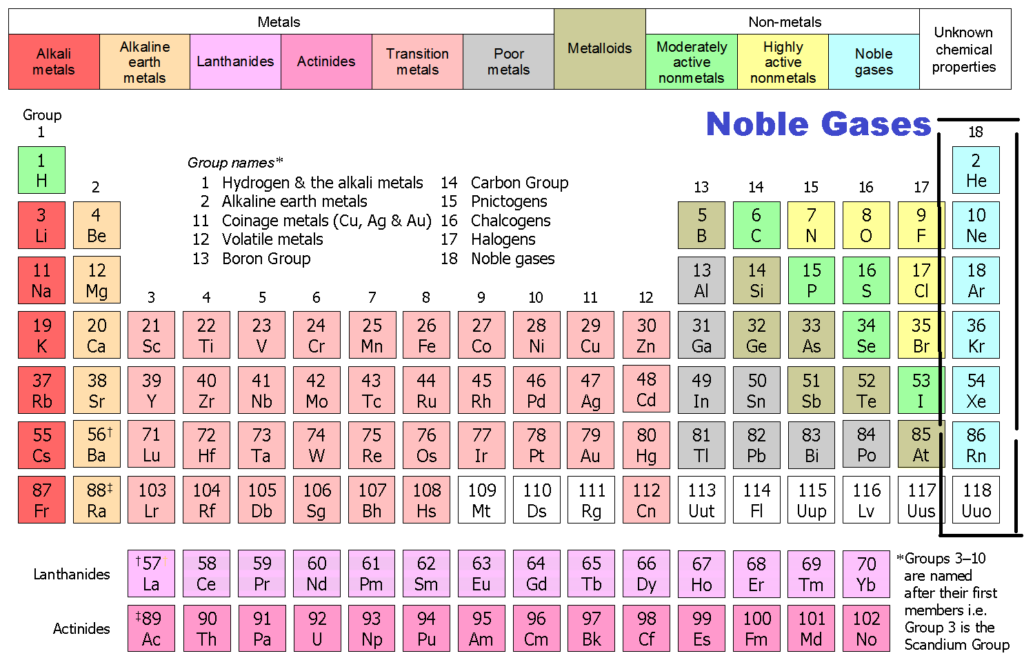
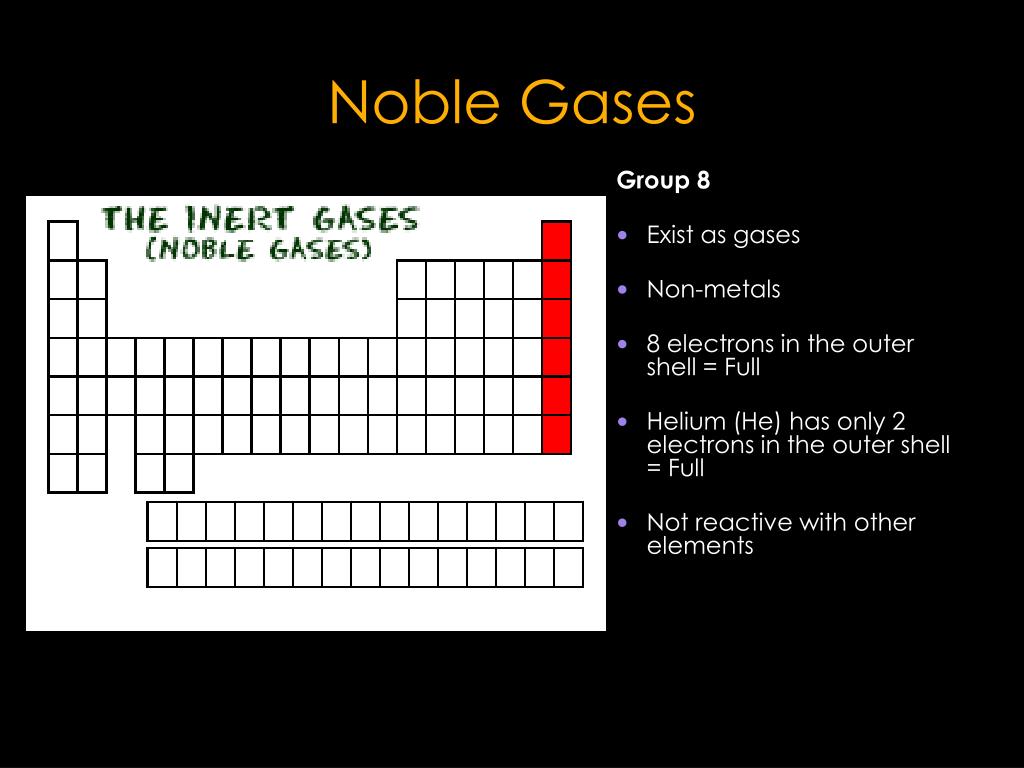
Group 18 The Noble Gases
Noble gases (ngs) are the least reactive elements in the periodic table towards chemical bond formation when compared with other elements because of. Another popular term is “noble gases,” suggesting that. 4/28/2022 wiki user ∙ 11y ago study now see answer (1) best answer copy the noble gasses. Web radon is radioactive and thereby is not used for decorative lighting..
Why Atoms Make Bonds Why Noble Gases are Stable Chemical Bonding
Web explain why noble gases are not likely to form chemical bonds. Because they are unreactive and have a full outer shell and don't need any electrons. Thus, they achieve a low energy level and are content without having to bond. The term stable in chemistry simply. 4/28/2022 wiki user ∙ 11y ago study now see answer (1) best answer.
PPT Matter PowerPoint Presentation, free download ID985294
Another popular term is “noble gases,” suggesting that. Web the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases due to the fact that their filled. Web why form chemical bonds? 2 people found it helpful. The force that hold two atoms together;
Question 4fd9e + Example
Another popular term is “noble gases,” suggesting that. The force that hold two atoms together; Because they are unreactive and have a full outer shell and don't need any electrons. Web stable atoms means no bonds can be formed, and since noble gases are stable, they cannot react. Web the noble gases (group 18) are located in the far right.
What Is The Reactivity Of Noble Gases howtogetalaid
2 people found it helpful. Web the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases due to the fact that their filled. Web stable atoms means no bonds can be formed, and since noble gases are stable, they cannot react. Because they already have an.
What Are Noble Gases? Definition and Properties
Web this group has been referred to as the “inert” gases, indicating that they are chemically inert, or unreactive. They have a full energy level discuss the formation. Web why form chemical bonds? Another popular term is “noble gases,” suggesting that. The force that hold two atoms together;
What's So Noble About Noble Gases? Owlcation Education
The force that hold two atoms together; Thus, they achieve a low energy level and are content without having to bond. 2 people found it helpful. Web noble gases, on the far right, already have full electron shells. Because they already have an electron configuration with a full, stable outer energy level.
Noble Gases Why Noble Gases are not Reactive Configuration by
Web stable atoms means no bonds can be formed, and since noble gases are stable, they cannot react. Many atoms become stable when their. Web why don't noble gasses tend to form chemical bonds? 2 people found it helpful. Noble gases have a full valence shell, which is why they rarely form bonds with other.
Because They Already Have An Electron Configuration With A Full, Stable Outer Energy Level.
Because they are unreactive and have a full outer shell and don't need any electrons. Web this group has been referred to as the “inert” gases, indicating that they are chemically inert, or unreactive. 2 people found it helpful. Another popular term is “noble gases,” suggesting that.
Noble Gases (Ngs) Are The Least Reactive Elements In The Periodic Table Towards Chemical Bond Formation When Compared With Other Elements Because Of.
In fact, they would lose something and likely have to go to a higher energy state in order to form a bond. Noble gases have a full valence shell, which is why they rarely form bonds with other. Many atoms become stable when their. Web the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases due to the fact that their filled.
Web Explain Why Noble Gases Are Not Likely To Form Chemical Bonds.
Web explain why noble gases are not likely to form chemical bonds because they already have a full octet so there less likely to react. Web therefore they're not going to gain anything by forming bonds. They have a full energy level discuss the formation. Web noble gases, on the far right, already have full electron shells.
4/28/2022 Wiki User ∙ 11Y Ago Study Now See Answer (1) Best Answer Copy The Noble Gasses.
Web why don't noble gasses tend to form chemical bonds? The force that hold two atoms together; Web stable atoms means no bonds can be formed, and since noble gases are stable, they cannot react. Web all noble gases have full s and p outer electron shells (except helium, which has no p sublevel), and so do not form chemical compounds easily.



.PNG)