Which Element Reacts With Oxygen To Form Ionic Bonds
Which Element Reacts With Oxygen To Form Ionic Bonds - Web up to 6% cash back sodium will react with oxygen and form an ionic compound. Web up to $3 cash back get the detailed answer: Lithium is in group 1 of the periodic table. Even though no oxides of krypton are known, oxygen is able to form. There are general trends in the reactions between main group elements and oxygen: 35 which element reacts with oxygen to form ionic bonds? 2) another example, magnesium and oxygen. A lithium atom will lose 1 electron to form a stable 1 + ion. Which element reacts with oxygen to form ionic bonds? Gains electrons and its radius gets.
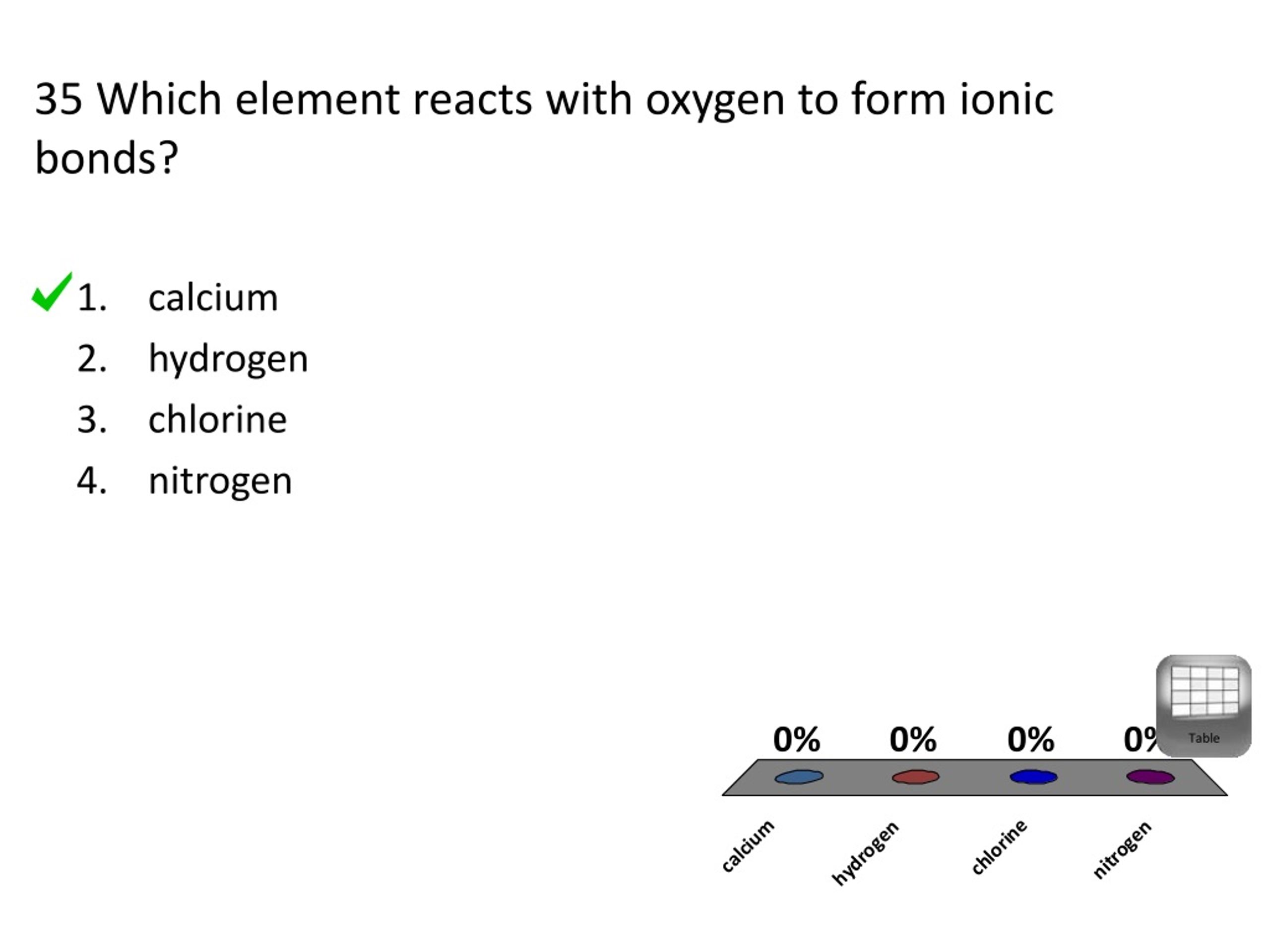
Web answered • expert verified. Web with metals, oxygen forms oxides that are largely ionic in character. (1) calcium (3) chlorine (2) hydrogen (4) nitrogen. Web up to 6% cash back sodium will react with oxygen and form an ionic compound. The two most basic types of. Which element reacts with oxygen to form ionic bonds? A lithium atom will lose 1 electron to form a stable 1 + ion. Web the positive/negative charge attraction would hold the two ions together. Web which element reacts with oxygen to form ionic bonds? The electronegativity of oxygen is 3.5 therefore any of the alkali or alkaline metals will ionically bond with oxygen to form an ionic compound.
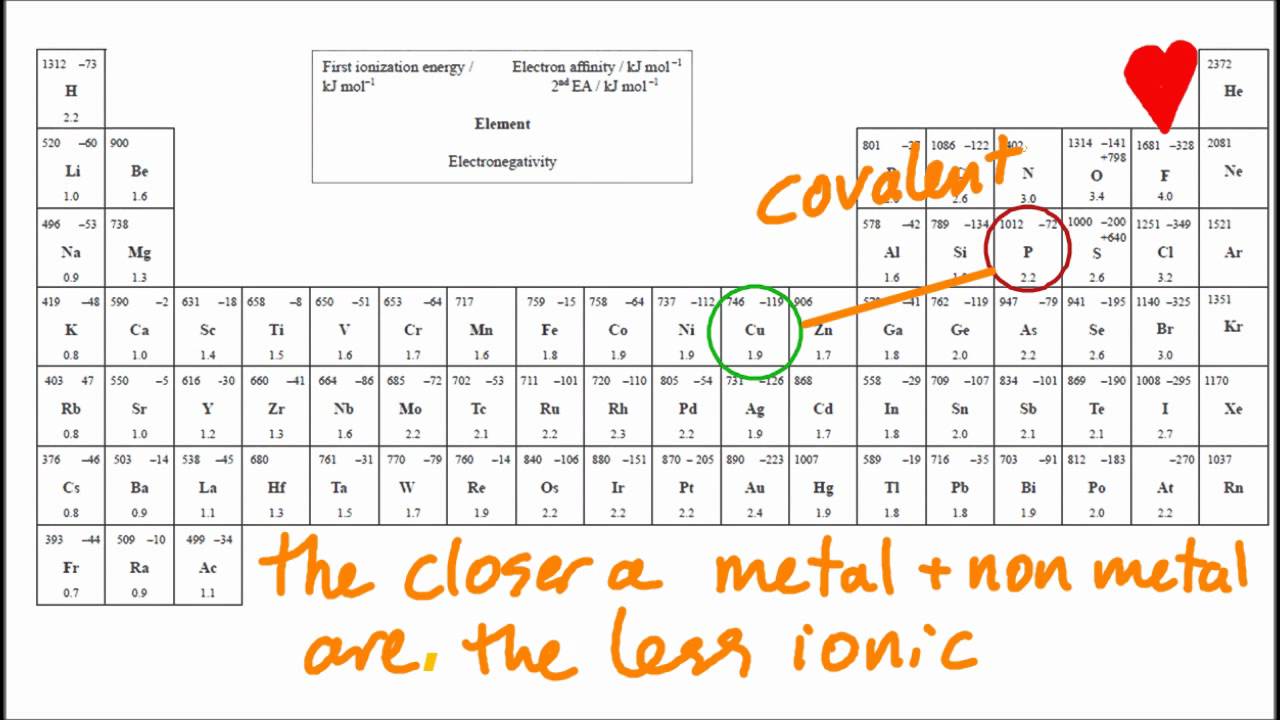
Based on electronegativity values, which type of elements tends to have the greatest attraction for electrons in a bond? Oxygen is in group 6 of the. Web the positive/negative charge attraction would hold the two ions together. (1) calcium (3) chlorine (2) hydrogen (4) nitrogen. Web molecular shape isomerism in organic compounds there are many types of chemical bonds and forces that bind molecules together. Which element reacts with oxygen to form ionic bonds? A metal and a nonmetal or polyatomic ions. Web according to the electronic configuration, elements with 1 or 2 valence electrons react with oxygen to form ionic bonds.what is electronic configuration?electronic Lithium is in group 1 of the periodic table. Web chemistry identify the element in period 3 of the periodic table that reacts with oxygen to form an ionic compound represented by the formula x2o.
4.2 Predict if a compound of 2 elements is ionic using the table of EN
(1) calcium (3) chlorine (2) hydrogen (4) nitrogen. A) calcium b)hydrogen c)chlorine d)nitrogen Web up to 6% cash back sodium will react with oxygen and form an ionic compound. Which of the following is false concerning this interaction? Web which element reacts with oxygen to form ionic bonds?
Sodium Reacts With Oxygen / Ionic compound for sodium oxide Science
35 which element reacts with oxygen to form ionic bonds? Oxygen is in group 6 of the. The two most basic types of. A metal and a nonmetal or polyatomic ions. Based on electronegativity values, which type of elements tends to have the greatest attraction for electrons in a bond?
Ionic Bond Definition, Types, Properties & Examples
35 which element reacts with oxygen to form ionic bonds? A lithium atom will lose 1 electron to form a stable 1 + ion. Web with metals, oxygen forms oxides that are largely ionic in character. Even though no oxides of krypton are known, oxygen is able to form. Based on electronegativity values, which type of elements tends to have.
Is SiO2 Ionic or Covalent? Techiescientist
Web answered • expert verified. 2) another example, magnesium and oxygen. Even though no oxides of krypton are known, oxygen is able to form. A metal and a nonmetal or polyatomic ions. Web the ionic bond formation for lithium oxide.
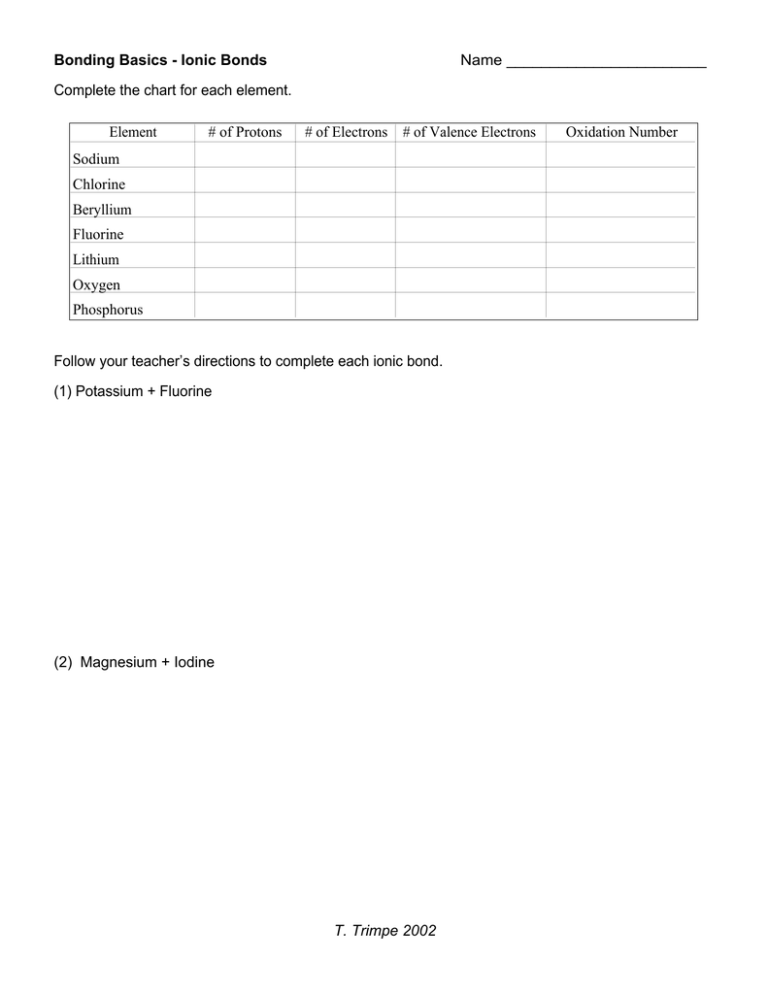
Bonding Basics Ionic Bonds Name _______________________ Element
Web answered • expert verified. A) calcium b)hydrogen c)chlorine d)nitrogen 35 which element reacts with oxygen to form ionic bonds? Web with metals, oxygen forms oxides that are largely ionic in character. Which element reacts with oxygen to form ionic bonds?
Solved Halogens can react with each other to form A)
Web the positive/negative charge attraction would hold the two ions together. An ionic bond by definition is the kind of chemical bond formed when there is a complete transfer of valence electrons from one. (1) calcium (3) chlorine (2) hydrogen (4) nitrogen. 2) another example, magnesium and oxygen. Web molecular shape isomerism in organic compounds there are many types of.
Is O2 ionic or covalent? Techiescientist
(1) calcium (3) chlorine (2) hydrogen (4) nitrogen. A lithium atom will lose 1 electron to form a stable 1 + ion. Web calcium reacts with oxygen to form an ionic bond. The two most basic types of. An ionic bond by definition is the kind of chemical bond formed when there is a complete transfer of valence electrons from.
chemistry Can carbon and titanium form an ionic bond
A lithium atom will lose 1 electron to form a stable 1 + ion. Web the positive/negative charge attraction would hold the two ions together. There are general trends in the reactions between main group elements and oxygen: Web up to $3 cash back get the detailed answer: 35 which element reacts with oxygen to form ionic bonds?
Oxygen form Periodic Table of Elements — Stock Photo © fambros 6284480
Web according to the electronic configuration, elements with 1 or 2 valence electrons react with oxygen to form ionic bonds.what is electronic configuration?electronic A) calcium b)hydrogen c)chlorine d)nitrogen Define ionic and molecular (covalent) compounds predict the type of compound formed from elements. Web which element reacts with oxygen to form ionic bonds? 8 months, 2 weeks ago.
PPT The University of the State of New York REGENTS HIGH SCHOOL
Web up to 6% cash back sodium will react with oxygen and form an ionic compound. (1) calcium (3) chlorine (2) hydrogen (4) nitrogen. Gains electrons and its radius gets. A) calcium b)hydrogen c)chlorine d)nitrogen An ionic compound is formed when there is a reaction between.
The Two Most Basic Types Of.
Lithium is in group 1 of the periodic table. Web calcium reacts with oxygen to form an ionic bond. Web which element reacts with oxygen to form ionic bonds? A) calcium b)hydrogen c)chlorine d)nitrogen
(1) Calcium (3) Chlorine (2) Hydrogen (4) Nitrogen.
There are general trends in the reactions between main group elements and oxygen: 8 months, 2 weeks ago. Web the positive/negative charge attraction would hold the two ions together. The magnesium would lose two electrons,.
Even Though No Oxides Of Krypton Are Known, Oxygen Is Able To Form.
An ionic compound is formed when there is a reaction between. Web learning objectives by the end of this section, you will be able to: A lithium atom will lose 1 electron to form a stable 1 + ion. Web according to the electronic configuration, elements with 1 or 2 valence electrons react with oxygen to form ionic bonds.what is electronic configuration?electronic
2) Another Example, Magnesium And Oxygen.
Web up to 6% cash back sodium will react with oxygen and form an ionic compound. Web up to $3 cash back get the detailed answer: An ionic bond by definition is the kind of chemical bond formed when there is a complete transfer of valence electrons from one. Web answered • expert verified.