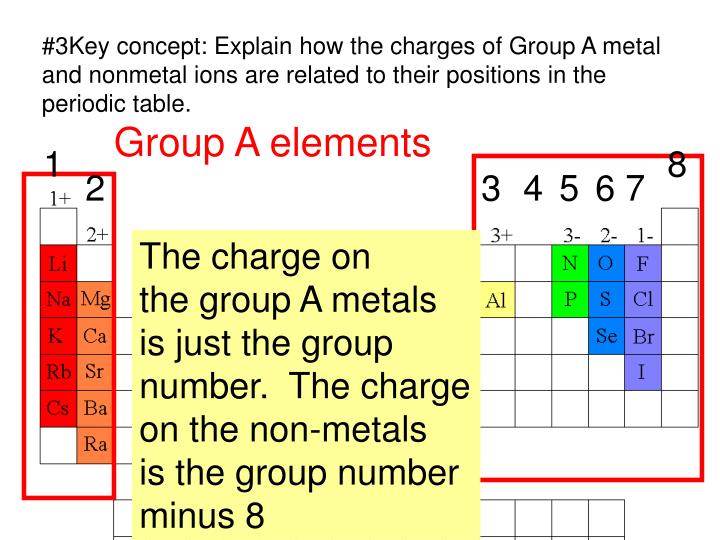
What Type Of Elements Form Anions
What Type Of Elements Form Anions - Web anions and cations hydrogen atom (center) contains a single proton and a single electron. Web halogens always form anions, alkali metals and alkaline earth metals always form cations. Web anions are a type of atom, the smallest particle of an element that still retains the element's properties. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Web metals with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble. Anions are negative ions that are formed when a nonmetal atom gains one or more electrons. Anions are so named because they are attracted to the anode (positive. Two types of ions can be created due to the gain and loss of electrons,.
Web anions are a type of atom, the smallest particle of an element that still retains the element's properties. Anions are so named because they are attracted to the anode (positive. Web forming negative ions (anions) atoms gain electrons in their outer shell when they form negative ions, called anions. Web negative ions, known as anions, form when an atom gains electrons and now has more electrons than protons, indicating that the number of protons and electrons is not. Atoms are made of three types of subatomic particles: Removal of the electron gives a cation (left), whereas the addition of an electron gives an. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Web there are two types of ions: Web anions and cations hydrogen atom (center) contains a single proton and a single electron. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet.
Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases. For example, sodium makes ionic compounds in which the sodium. Web metals with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Atoms are made of three types of subatomic particles: Web most of the elements that make ionic compounds form an ion that has a characteristic charge. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Web some common elements that form anions are hydrogen, fluorine, iodine and oxygen. Anions are so named because they are attracted to the anode (positive. Removal of the electron gives a cation (left), whereas the addition of an electron gives an. Web negative ions, known as anions, form when an atom gains electrons and now has more electrons than protons, indicating that the number of protons and electrons is not.
PPT Naming Compounds PowerPoint Presentation ID3350086
Web anions and cations hydrogen atom (center) contains a single proton and a single electron. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Web for elements in groups. Web moving from the far right to the left on the periodic table, elements often form anions with a.
Naming Simple Ionic Compounds Pathways to Chemistry
A sodium atom loses one electron to form a sodium ion forming negative ions Atoms are made of three types of subatomic particles: Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble. Atoms of group 16 gain.
The anion of an element has YouTube
For example, sodium makes ionic compounds in which the sodium. Two types of ions can be created due to the gain and loss of electrons,. Web atoms of group 17 gain one electron and form anions with a 1− charge; Web most of the elements that make ionic compounds form an ion that has a characteristic charge. Anions are negative.
anion Common anions, their names, formulas and the elements they are
A sodium atom loses one electron to form a sodium ion forming negative ions Web metals with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Web most of.
Which Group of Elements can Form Anions Most Quickly?
Anions are so named because they are attracted to the anode (positive. Web halogens always form anions, alkali metals and alkaline earth metals always form cations. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble. Web negative.
Image result for cations vs anions Chemistry classroom, Electron
Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Web most of the elements that make ionic compounds form an ion that has a characteristic charge. A sodium atom loses one electron to form a sodium ion forming negative ions Web anions and cations hydrogen atom (center) contains a single proton and.
PPT 1 Name the ions formed by these elements and classify them as
Anions are so named because they are attracted to the anode (positive. Web most of the elements that make ionic compounds form an ion that has a characteristic charge. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the.
PPT IONS PowerPoint Presentation, free download ID2435906
Web some common elements that form anions are hydrogen, fluorine, iodine and oxygen. Web negative ions, known as anions, form when an atom gains electrons and now has more electrons than protons, indicating that the number of protons and electrons is not. Web there are two types of ions: Web anions are a type of atom, the smallest particle of.
PPT Chapter 7 Chemical Formulas and Chemical Compounds PowerPoint
These ions are negative because they contain more. Oxygen needs to gain two electrons so it forms an anion with a charge of. An anion has a net negative. Web most of the elements that make ionic compounds form an ion that has a characteristic charge. Removal of the electron gives a cation (left), whereas the addition of an electron.
PPT 1 Name the ions formed by these elements and classify them as
Web atoms of group 17 gain one electron and form anions with a 1− charge; Removal of the electron gives a cation (left), whereas the addition of an electron gives an. Web most of the elements that make ionic compounds form an ion that has a characteristic charge. A cation has a net positive electrical charge, which means it has.
Atoms Of Group 16 Gain Two Electrons And Form Ions With A 2− Charge, And So On.
Web anions are a type of atom, the smallest particle of an element that still retains the element's properties. A sodium atom loses one electron to form a sodium ion forming negative ions Web some common elements that form anions are hydrogen, fluorine, iodine and oxygen. Web for elements in groups.
Anions Are So Named Because They Are Attracted To The Anode (Positive.
These ions are negative because they contain more. Web forming negative ions (anions) atoms gain electrons in their outer shell when they form negative ions, called anions. A cation has a net positive electrical charge, which means it has more protons than electrons. Web 162k views types of ions:
Removal Of The Electron Gives A Cation (Left), Whereas The Addition Of An Electron Gives An.
1, 2 and 3, the number of electrons lost is the same as the group number. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Web anions and cations hydrogen atom (center) contains a single proton and a single electron. Web halogens always form anions, alkali metals and alkaline earth metals always form cations.
Atoms Are Made Of Three Types Of Subatomic Particles:
An anion has a net negative. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble. Cations and anions to create an ion, atoms gain or lose electrons. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases.