What Kinds Of Elements Form Ionic Compounds
What Kinds Of Elements Form Ionic Compounds - Part a when a metal and a nonmetal exchange electrons to make a cation and an anion, respectively, they can form an ionic. Composition of ions exercise 3.5. Ionic compounds generally form between elements that are metals and elements that are nonmetals. Web which types of elements combine to form ionic compounds? Web polar covalent compounds which had put with ionic compounds in the same water will form into the electrolyte solution. Web ionic compounds containing basic ions hydroxide (oh −) or oxide (o 2−) are classified as bases. Web p olyatomic ions can bond with monatomic ions or with other polyatomic ions to form compounds. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between. Web give an example of the formation of an ionic compound from its elements answer in ionic bonding, a metal and a nonmetal react to form oppositely charged ions (electrons are. Metals often react with nonmetals to form ionic compounds.
Ionic compounds without these ions are also known as salts and can be formed. The two most basic types of bonds are characterized as either ionic or. Web ionic compounds form when positive and negative ions share electrons and form an ionic bond. In order to form neutral compounds, the total charges must be balanced. Web which types of elements combine to form ionic compounds? Web there are many types of chemical bonds and forces that bind molecules together. Web p olyatomic ions can bond with monatomic ions or with other polyatomic ions to form compounds. The o atom in water has a partial negative charge. Web answer (1 of 9): Formation of ions exercise 3.5.
The h atoms have a partial. Web expert answer transcribed image text: Composition of ions exercise 3.5. In order to form neutral compounds, the total charges must be balanced. Web polar covalent compounds which had put with ionic compounds in the same water will form into the electrolyte solution. Ionic compounds generally form between elements that are metals and elements that are nonmetals. Web give an example of the formation of an ionic compound from its elements answer in ionic bonding, a metal and a nonmetal react to form oppositely charged ions (electrons are. Elements with surplus of electrons in their outermost orbit give their. The o atom in water has a partial negative charge. Web ionic compounds form when positive and negative ions share electrons and form an ionic bond.
Naming Simple Ionic Compounds Pathways to Chemistry
Formation of ions exercise 3.5. Metal + nonmetal → ionic compound metal + polyatomic ion → ionic compound; Web an ionic bond is a type ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two. Web there are many types of chemical bonds and forces that bind molecules together..
Ionic bonding Wikipedia
Web anios example 3.5. Elements with surplus of electrons in their outermost orbit give their. Web ionic compounds form by two ways: In order to form neutral compounds, the total charges must be balanced. Web which types of elements combine to form ionic compounds?
Ionic Bond Definition, Types, Properties & Examples
Ionic compounds without these ions are also known as salts and can be formed. Ionic compounds generally form between elements that are metals and elements that are nonmetals. Predict whether each pair would form ionic bonds. Elements with surplus of electrons in their outermost orbit give their. The two most basic types of bonds are characterized as either ionic or.
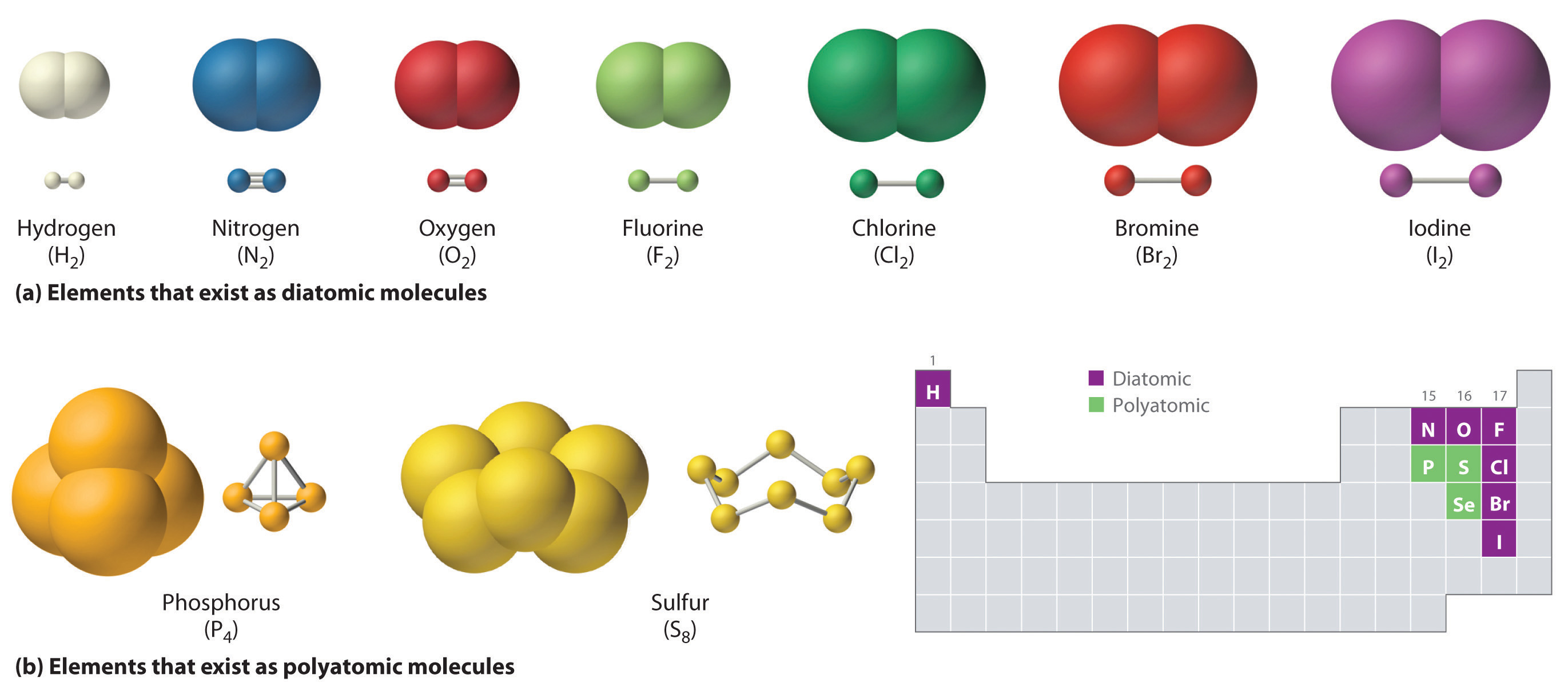
2.6 Molecules and Molecular Compounds Chemistry LibreTexts
The o atom in water has a partial negative charge. Web answer (1 of 9): Metals often react with nonmetals to form ionic compounds. Web the elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin,. Web give an example of the formation of an ionic compound from its elements.
10 Notable Differences Between Ionic And Covalent Bonds Current
Web expert answer transcribed image text: Ionic compounds generally form between elements that are metals and elements that are nonmetals. 2 polyatomic ions ionic compounds example 3.5. Web ionic compounds containing basic ions hydroxide (oh −) or oxide (o 2−) are classified as bases. Predict whether each pair would form ionic bonds.
Ionic Bond Definition, Types, Properties & Examples
Web expert answer transcribed image text: Predict whether each pair would form ionic bonds. Elements with surplus of electrons in their outermost orbit give their. Web an ionic bond is a type ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two. Ionic compounds generally form between elements that.
Ionic Bond Definition Easy Hard Science
The two most basic types of bonds are characterized as either ionic or. Ionic compounds generally form between elements that are metals and elements that are nonmetals. Web anios example 3.5. Web ionic compounds containing basic ions hydroxide (oh −) or oxide (o 2−) are classified as bases. Metal + nonmetal → ionic compound metal + polyatomic ion → ionic.
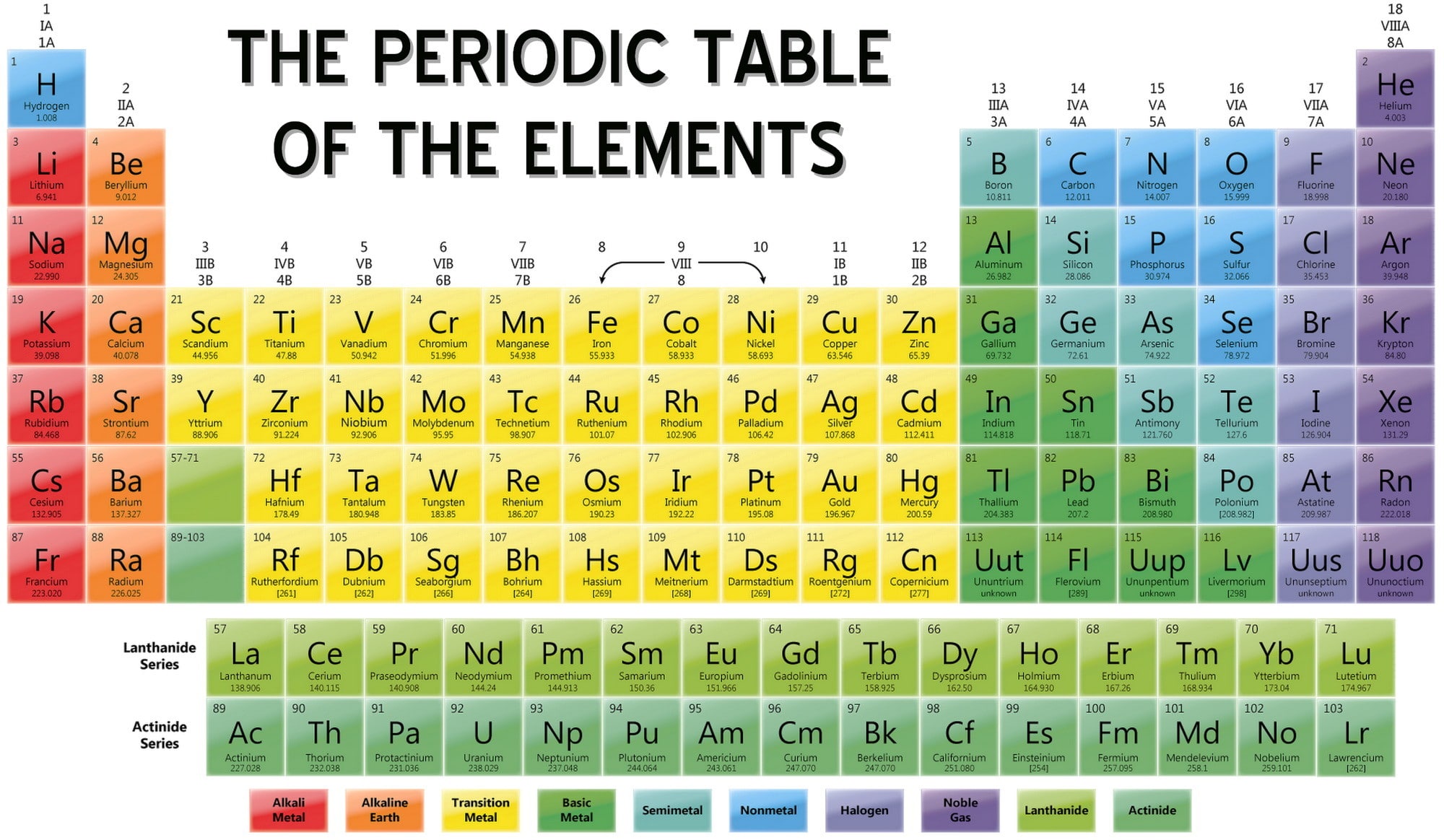
Periodic Table Ions List Periodic Table Timeline
Web ionic compounds containing basic ions hydroxide (oh −) or oxide (o 2−) are classified as bases. Web answer (1 of 9): Web give an example of the formation of an ionic compound from its elements answer in ionic bonding, a metal and a nonmetal react to form oppositely charged ions (electrons are. Ionic compounds generally form between elements that.
Periodic Table of Elements & Ionic Bonding Mrs. Zeringue's 7th Grade
Look at each of the following pairs of elements. Web polar covalent compounds which had put with ionic compounds in the same water will form into the electrolyte solution. The h atoms have a partial. Web ionic compounds form by two ways: Composition of ions exercise 3.5.
Examples of Ionic Bonds and Compounds
Part a when a metal and a nonmetal exchange electrons to make a cation and an anion, respectively, they can form an ionic. Web what kinds of elements combine to form ionic compounds? Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between. Web ionic compounds form when positive and negative ions share electrons and form.
Web Which Types Of Elements Combine To Form Ionic Compounds?
Part a when a metal and a nonmetal exchange electrons to make a cation and an anion, respectively, they can form an ionic. Generally ionic compounds are formed as a result of transfer of electrons between elements. Web polar covalent compounds which had put with ionic compounds in the same water will form into the electrolyte solution. Elements combine to form chemical compounds that are often divided into two categories.
Ionic Compounds Generally Form Between Elements That Are Metals And Elements That Are Nonmetals.
Web anios example 3.5. Web when electrons are transferred and ions form, ionic bonds result. Web question #975aa ionic compounds usually dissociate in water because water is a polar molecule. Predict whether each pair would form ionic bonds.
The O Atom In Water Has A Partial Negative Charge.
Web ionic compounds form when positive and negative ions share electrons and form an ionic bond. Web answer (1 of 9): The h atoms have a partial. Web what kinds of elements combine to form ionic compounds?
Web Expert Answer Transcribed Image Text:
Web ionic compounds containing basic ions hydroxide (oh −) or oxide (o 2−) are classified as bases. The strong attraction between positive and negative ions often. Web the elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin,. 2 polyatomic ions ionic compounds example 3.5.







/ionic-bond-58fd4ea73df78ca1590682ad.jpg)