What Are The 4 Types Of Bonds Carbon Can Form
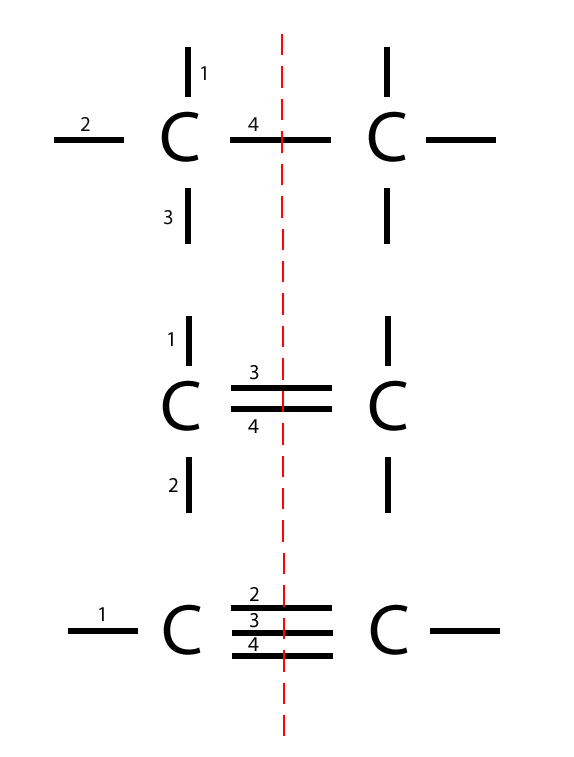
What Are The 4 Types Of Bonds Carbon Can Form - Web what 4 types of bonds carbon can form? Web carbon atoms can also form double bonds in compounds called alkenes or triple bonds in compounds called alkynes. Web carbon can form single bonds (sharing of 2 electrons), double bonds (sharing of 4 electrons), and/or a triple bond (sharing of 6 electrons). Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Sp^3 is where the four bonds possible with a carbon atom are hybridized from one s orbital and 3 p orbitals into four. Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. Solution verified answered this week create an account to view solutions To form ionic bonds, carbon molecules must either gain or. Web the four types of bonds that carbon can form are as follows: Single bond, double bond, triples bonds, and or quadruple bonds.
Name the 4 types of bonds carbon can form. In a polar covalent bond, the electrons are unequally shared by the atoms and spend more time close to one atom. It is nonmetallic and tetravalent —its atom making four electrons available to. Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. Carbon shares electrons with other atoms. Solution verified answered this week create an account to view solutions Web carbon atoms can also form double bonds in compounds called alkenes or triple bonds in compounds called alkynes. Web the four types of bonds that carbon can form are as follows: To form ionic bonds, carbon molecules must either gain or. The electronegativity of carbon ( en = 2.55) is too small to allow carbon.
Web there are two basic types of covalent bonds: Web name the 4 main elements that make up 95% of an organism. Sp^3 is where the four bonds possible with a carbon atom are hybridized from one s orbital and 3 p orbitals into four. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. To form ionic bonds, carbon molecules must either gain or. It is nonmetallic and tetravalent —its atom making four electrons available to. Web the most common bond carbon can form is a covalent bond. Web what 4 types of bonds carbon can form? The simplest carbon molecule is methane (ch 4 ), depicted here. Web answer (1 of 9):
Quiz & Worksheet What Types of Bonds Can Carbon Form?
Carbon shares electrons with other atoms. In a polar covalent bond, the electrons are unequally shared by the atoms and spend more time close to one atom. Web carbon atoms can also form double bonds in compounds called alkenes or triple bonds in compounds called alkynes. There are four types of carbon bonds. Carbon does not form ionic bonds because.
The 4 Types of Bonds Carbon Can Form Video & Lesson Transcript
Web answer (1 of 9): Single bond, double bond, triples bonds, and or quadruple bonds. Carbon can form four covalent bonds to create an organic molecule. Sp^3 is where the four bonds possible with a carbon atom are hybridized from one s orbital and 3 p orbitals into four. Web the four bonds carbon can make are:
Chemical Bonds Definition, Types, and Examples
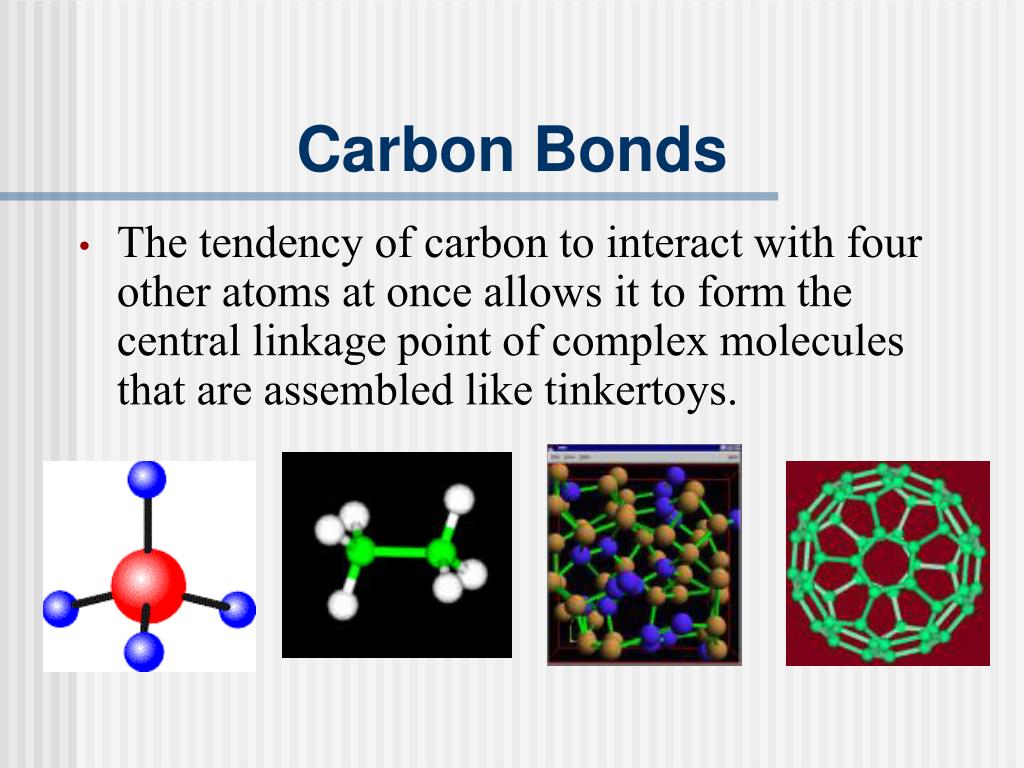
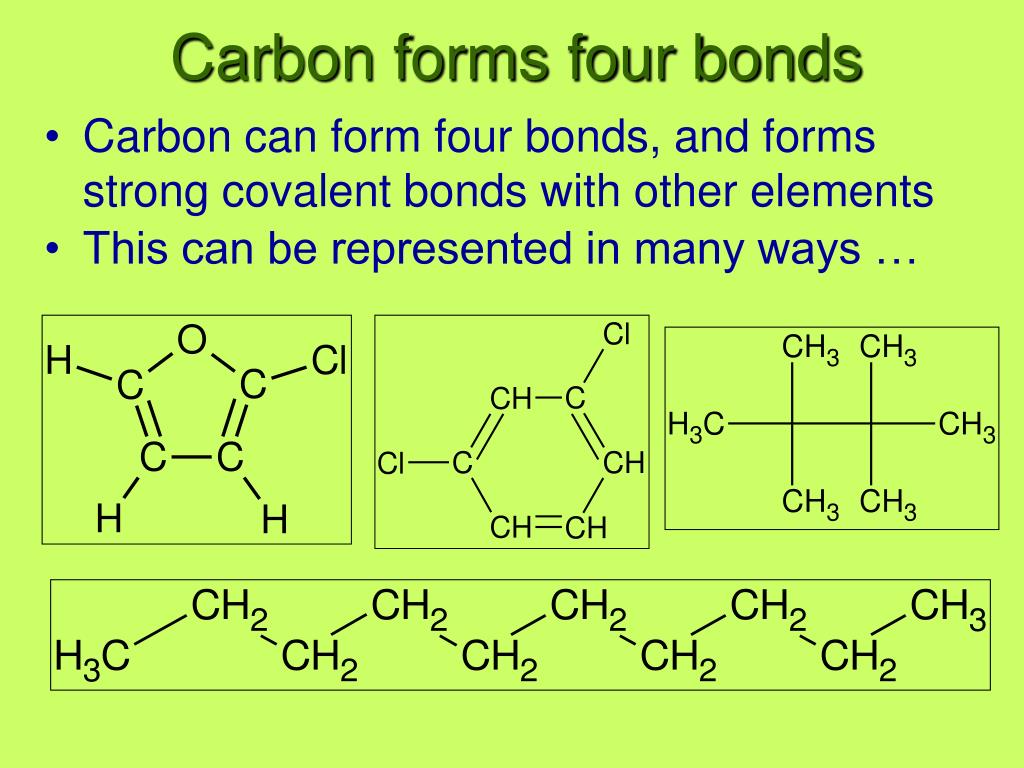
Carbon can form four covalent bonds to create an organic molecule. Web the four types of bonds that carbon can form are as follows: Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. Single bond, double bond, triples bonds, and or quadruple bonds. Web the four bonds carbon can make are:
PPT Biochemistry PowerPoint Presentation, free download ID89333
Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. Single bond, double bond, triples bonds, and or quadruple bonds. Sp^3 is where the four bonds possible with a carbon atom are hybridized from one s orbital and 3 p orbitals into four. It is nonmetallic and tetravalent —its atom making four electrons available.
Answered 7.10. Carbon can form bonds with itself… bartleby
Web the four bonds carbon can make are: The electronegativity of carbon ( en = 2.55) is too small to allow carbon. What three types of covalent bonds does carbon form?. Web carbon carbon (from latin carbo 'coal') is a chemical element with the symbol c and atomic number 6. Web the most common bond carbon can form is a.
PPT CHAPTER 25 CARBON AND ITS COMPOUNDS PowerPoint Presentation
The simplest carbon molecule is methane (ch 4 ), depicted here. Carbon can form four covalent bonds to create an organic molecule. Web the four types of bonds that carbon can form are as follows: To form ionic bonds, carbon molecules must either gain or. Web moreover, of all the elements in the second row, carbon has the maximum number.
duermen los delfines en la citacion? MNC COURIER
Solution verified answered this week create an account to view solutions Web what 4 types of bonds carbon can form? To form ionic bonds, carbon molecules must either gain or. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). Web moreover,.
PPT Biochemistry PowerPoint Presentation, free download ID89333
Sp^3 is where the four bonds possible with a carbon atom are hybridized from one s orbital and 3 p orbitals into four. What three types of covalent bonds does carbon form?. Web the four bonds carbon can make are: Web answer (1 of 9): Web the most common bond carbon can form is a covalent bond.
PPT Organic Chemistry PowerPoint Presentation, free download ID5887001
Carbon can form four covalent bonds to create an organic molecule. To form ionic bonds, carbon molecules must either gain or. Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. The electronegativity of carbon ( en = 2.55) is too small to allow carbon. It is nonmetallic and tetravalent —its atom making four.
Carbon to Carbon Single, Double & Triple Bonds Surfguppy
Web the most common bond carbon can form is a covalent bond. Web name the 4 main elements that make up 95% of an organism. Name the 4 types of bonds carbon can form. Web what 4 types of bonds carbon can form? Solution verified answered this week create an account to view solutions
There Are Four Types Of Carbon Bonds.
Web carbon carbon (from latin carbo 'coal') is a chemical element with the symbol c and atomic number 6. Sp^3 is where the four bonds possible with a carbon atom are hybridized from one s orbital and 3 p orbitals into four. Web carbon can form single bonds (sharing of 2 electrons), double bonds (sharing of 4 electrons), and/or a triple bond (sharing of 6 electrons). Single bond, double bond, triples bonds, and or quadruple bonds.
The Simplest Carbon Molecule Is Methane (Ch 4 ), Depicted Here.
Web answer (1 of 9): The electronegativity of carbon ( en = 2.55) is too small to allow carbon. Web the four types of bonds that carbon can form are as follows: Web what 4 types of bonds carbon can form?
Web The Most Common Bond Carbon Can Form Is A Covalent Bond.
Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). Web carbon atoms can also form double bonds in compounds called alkenes or triple bonds in compounds called alkynes. What three types of covalent bonds does carbon form?. Solution verified answered this week create an account to view solutions
Carbon Shares Electrons With Other Atoms.
To form ionic bonds, carbon molecules must either gain or. Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. In a polar covalent bond, the electrons are unequally shared by the atoms and spend more time close to one atom. Carbon can form four covalent bonds to create an organic molecule.