How Many Bonds Can N Form
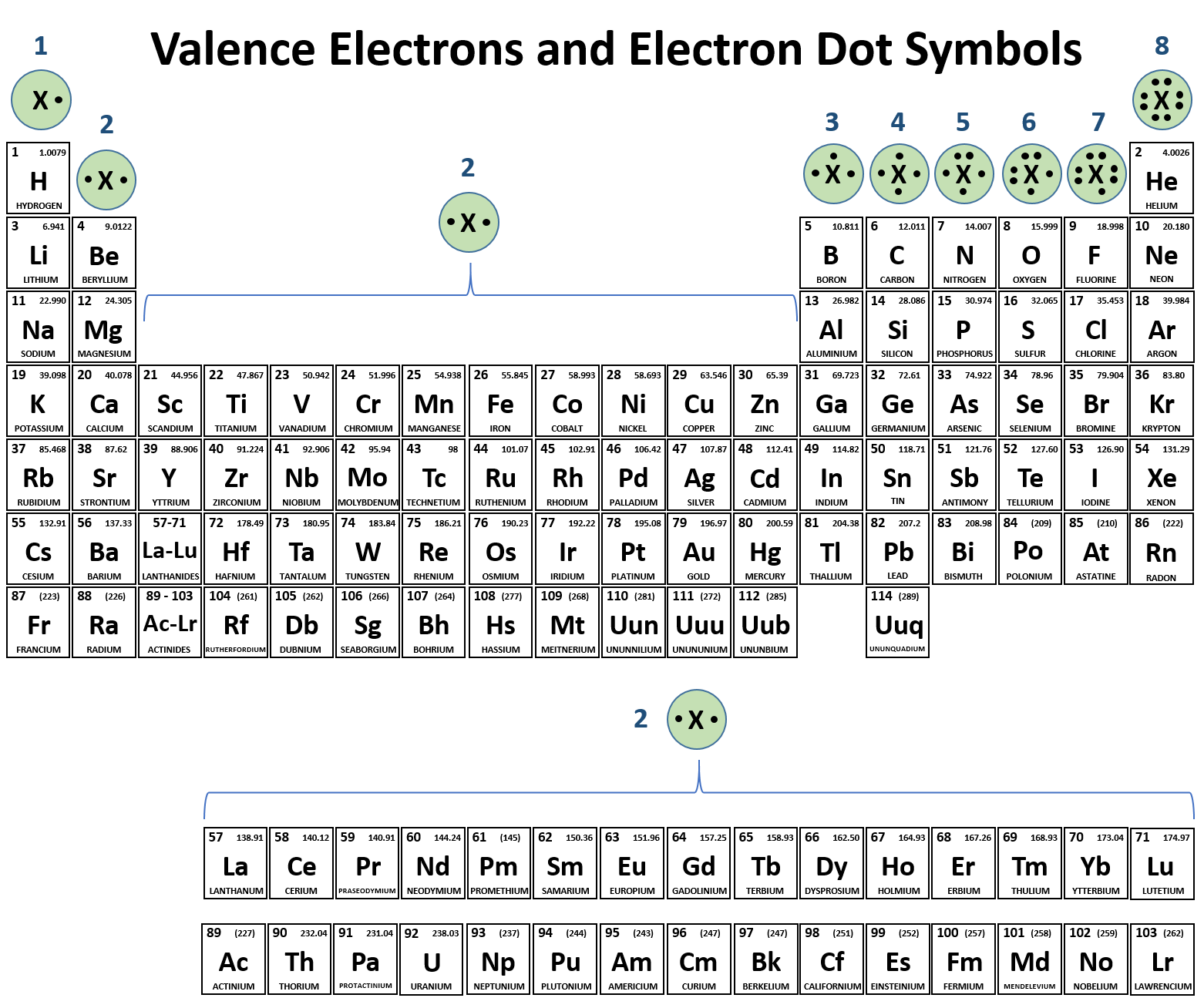
How Many Bonds Can N Form - The electrons involved are in the outer shells of the atoms. Group 6a form 2 bonds; Group 5a form 3 bonds; How many types of bonds can atom form? In general, it depends on how many electrons it has or are missing in the outermost electron shell. Web how many bonds can an atom form? How many types of bonds can atom form? Draw dot diagrams for s and for. Which of the following species cannot exist? Web as for the formation of a single covalent bond, one electron is shared by each atom, thus nitrogen can share its three electrons to form 3 single covalent bonds.
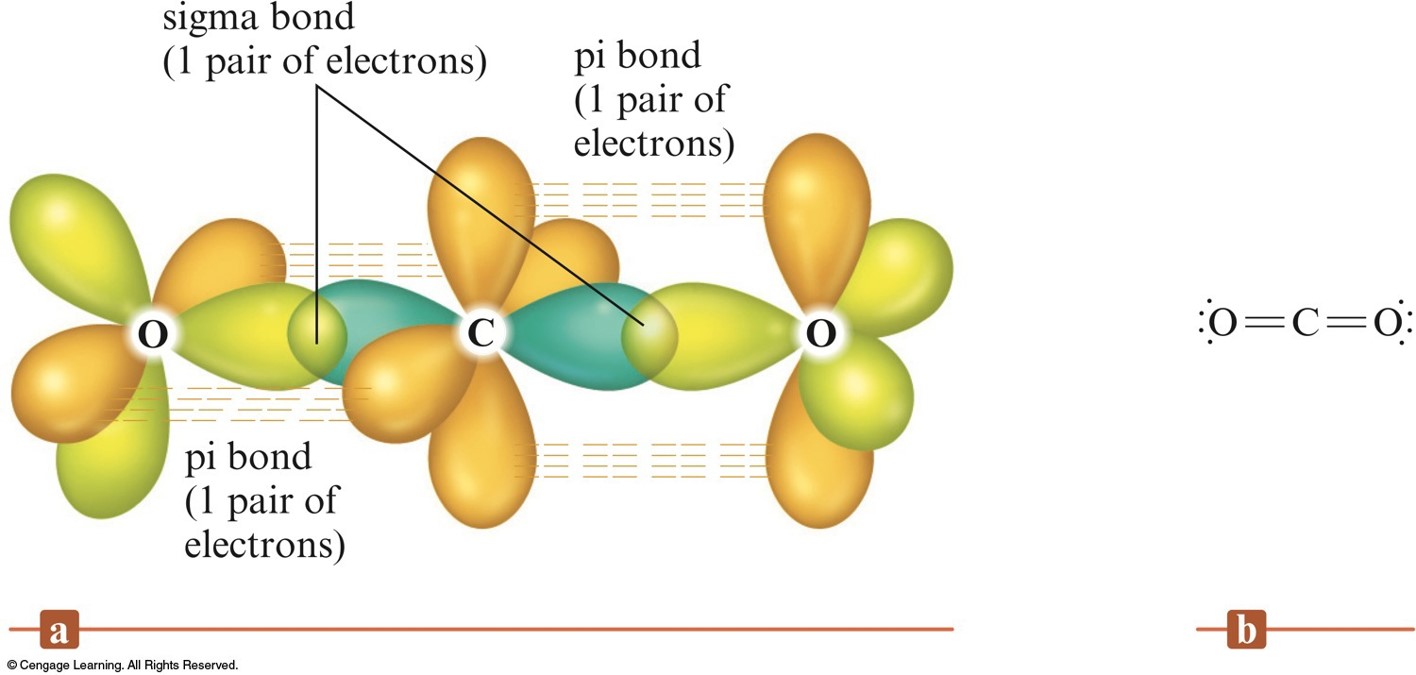
Web but you can approximate the yield to maturity with the following shortcut formula: Annual interest + annually accumulated discount/ average of par value and. Web study with quizlet and memorize flashcards containing terms like how many bonds can n form?, primary amine, secondary amine and more. Web typically, the atoms of group 4a form 4 covalent bonds; Group 6a form 2 bonds; Group 5a form 3 bonds; Carbon form generally covalent bonds; And group 7a form one bond. Web the two n atoms are bonded together by a triple bond, consisting of a σ and two π bonds. Web typically, the atoms of group 4a form 4 covalent bonds;
Web how many bonds can an atom form? The central atom n (group 5a) has 3 bonds and one lone pair. Web but you can approximate the yield to maturity with the following shortcut formula: Web for covalent bonds, it can form two single bonds or one double bond. Web both cl and n form the expected number of bonds. In general, it depends on how many electrons it has or are missing in the outermost electron shell. Web typically, the atoms of group 4a form 4 covalent bonds; Group 5a form 3 bonds; Web chemistry chemistry questions and answers 1. The electrons involved are in the outer shells of the atoms.
Reading Covalent Bonds Biology I
Group 6a form 2 bonds; And group 7a form one bond. N 2 is a stable (that is relatively unreactive) molecular compound. Web as for the formation of a single covalent bond, one electron is shared by each atom, thus nitrogen can share its three electrons to form 3 single covalent bonds. Recognising where there are lone pairs that.
How many pi bonds can the atom form? Socratic
Group 6a form 2 bonds; Web for covalent bonds, it can form two single bonds or one double bond. Web as for the formation of a single covalent bond, one electron is shared by each atom, thus nitrogen can share its three electrons to form 3 single covalent bonds. Web but you can approximate the yield to maturity with the.
__TOP__ How Many Covalent Bonds Can Chlorine Form
How many bonds does each of the following atoms normally form in molecules? Group 6a form 2 bonds; H c n f 2. Web generally, the greater the risk, the higher the interest paid by a bond. Web both cl and n form the expected number of bonds.
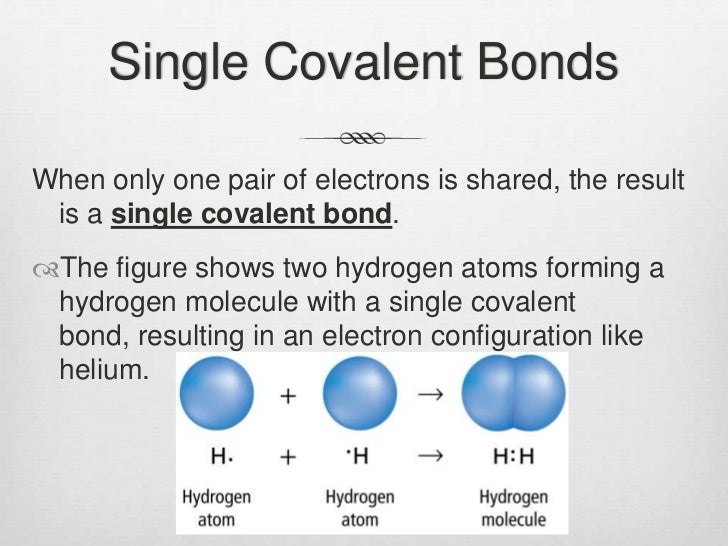
How many covalent bonds can hydrogen form?
Web but you can approximate the yield to maturity with the following shortcut formula: Annual interest + annually accumulated discount/ average of par value and. Group 5a form 3 bonds; Recognising where there are lone pairs that. Which of the following species cannot exist?
The 4 Types of Bonds Carbon Can Form Video & Lesson Transcript
This is called the valence shell. Returns on bonds are usually lower than those of stocks, but the. Web as for the formation of a single covalent bond, one electron is shared by each atom, thus nitrogen can share its three electrons to form 3 single covalent bonds. H c n f 2. The central atom n (group 5a) has.
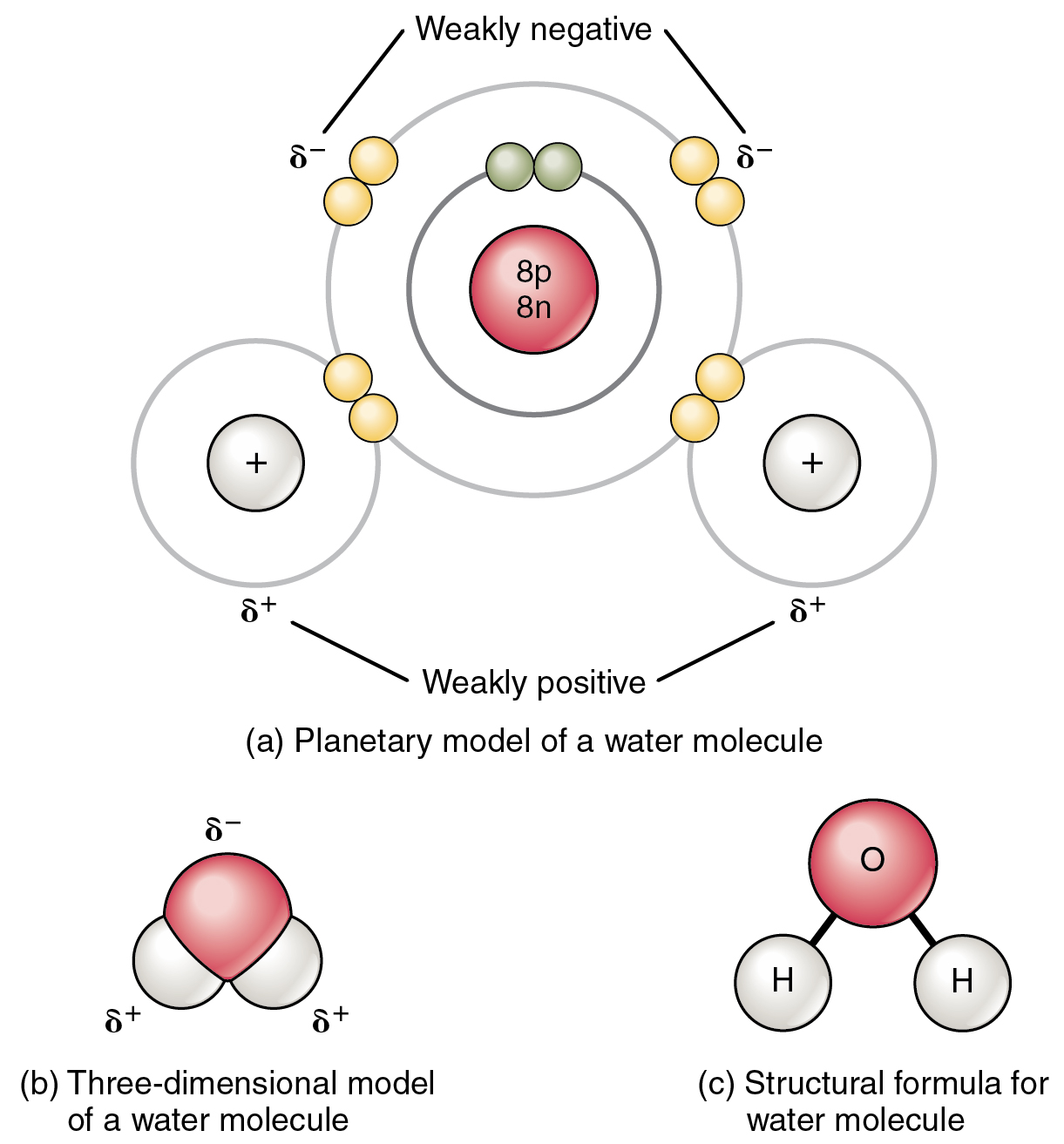
Chemical Bonds · Anatomy and Physiology
The number of electrons required to obtain. Web for covalent bonds, it can form two single bonds or one double bond. Group 6a form 2 bonds; Web both cl and n form the expected number of bonds. Group 5a form 3 bonds;
polarity Definition & Examples Britannica
Group 6a form 2 bonds; In general, it depends on how many electrons it has or are missing in the outermost electron shell. Returns on bonds are usually lower than those of stocks, but the. This is called the valence shell. And group 7a form one bond.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
And group 7a form one bond. The number of electrons required to obtain. Hydrogen has one electron in its. Group 6a form 2 bonds; Web as for the formation of a single covalent bond, one electron is shared by each atom, thus nitrogen can share its three electrons to form 3 single covalent bonds.
metallic bond Properties, Examples, & Explanation Britannica
Web both cl and n form the expected number of bonds. Web for covalent bonds, it can form two single bonds or one double bond. Web the two n atoms are bonded together by a triple bond, consisting of a σ and two π bonds. Which of the following species cannot exist? Annual interest + annually accumulated discount/ average of.
Chemical Bonds Anatomy and Physiology I
In general, it depends on how many electrons it has or are missing in the outermost electron shell. Returns on bonds are usually lower than those of stocks, but the. Draw dot diagrams for s and for. Group 6a form 2 bonds; Web as for the formation of a single covalent bond, one electron is shared by each atom, thus.
Carbon Form Generally Covalent Bonds;
Web both cl and n form the expected number of bonds. Web typically, the atoms of group 4a form 4 covalent bonds; N 2 is a stable (that is relatively unreactive) molecular compound. This is called the valence shell.
Web How Many Bonds Can An Atom Form?
The number of electrons required to obtain. Web but you can approximate the yield to maturity with the following shortcut formula: And group 7a form one bond. H c n f 2.
Group 6A Form 2 Bonds;
Group 6a form 2 bonds; Recognising where there are lone pairs that. Web the two n atoms are bonded together by a triple bond, consisting of a σ and two π bonds. In general, it depends on how many electrons it has or are missing in the outermost electron shell.
Web Typically, The Atoms Of Group 4A Form 4 Covalent Bonds;
Web generally, the greater the risk, the higher the interest paid by a bond. Annual interest + annually accumulated discount/ average of par value and. Web as for the formation of a single covalent bond, one electron is shared by each atom, thus nitrogen can share its three electrons to form 3 single covalent bonds. Group 5a form 3 bonds;