How Many Bonds Can A Hydrogen Atom Form
How Many Bonds Can A Hydrogen Atom Form - 1 (apex) how many covalent bonds will a hydrogen atom normally make? This is the reason why h is always a terminal atom and never a central atom. 100% (1 rating) valence of hydrogen atom is 1. Web for example, in methane (ch _4 4 ), carbon forms covalent bonds with four hydrogen atoms. Each bond corresponds to a pair of shared electrons (one from carbon and one. There are exactly the right numbers of + hydrogens. Web how many bonds can a hydrogen atom form? Web what type of bond can hydrogen atom form? A hydrogen atom will typically form two. A hydrogen bond is a kind of bonding that is present between an atom of.
Which of the following species cannot exist? A hydrogen bond is a kind of bonding that is present between an atom of. Web apart from some group 13 weirdness, hydrogen can only make one bond. Hydrogen bonds with hydrogen bond acceptor atoms such as oxygen. Each bond corresponds to a pair of shared electrons (one from carbon and one. 100% (1 rating) valence of hydrogen atom is 1. Web how many bonds can an atom form? A hydrogen atom will typically form two. Web hydrogen only needs to form one bond to complete a duet of electrons. Web since hydrogen has only one valence electron, it will only bond once.
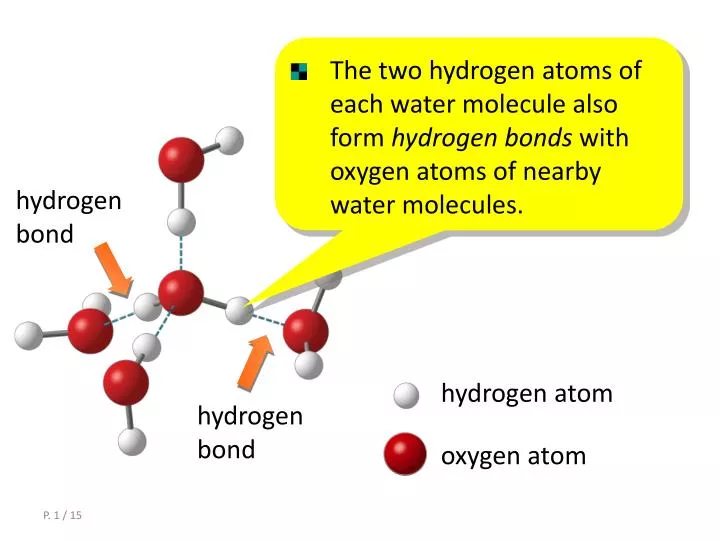
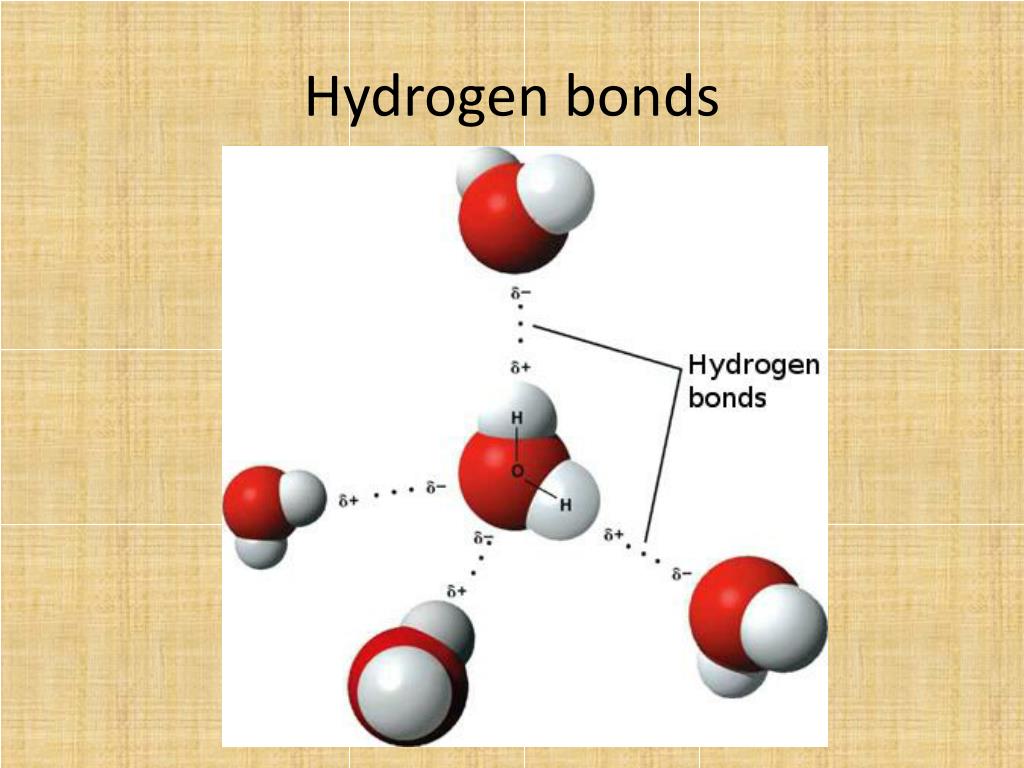
Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. That is why, one electron present in the hydrogen atom can form a covalent bond with the unpaired electron of. There are exactly the right numbers of + hydrogens. Web how many bonds can a hydrogen atom form? Web what type of bond can hydrogen atom form? 1 (apex) how many covalent bonds will a hydrogen atom normally make? A hydrogen bond is a kind of bonding that is present between an atom of. Web how many bonds can an atom form? Web since hydrogen has only one valence electron, it will only bond once. This is the reason why h is always a terminal atom and never a central atom.
Bonds That Hold Water Molecules Together / Intermolecular Forces
Web cbse notes live join vedantu’s free mastercalss what is a hydrogen bond? Thus one hydrogen atom will only bond once. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Each bond corresponds to a pair of shared electrons (one from carbon and one. Web hydrogen only needs to form one bond to.
How many hydrogen bonds a water molecule can form Hydrogen Bonding in
Covalent bonds with nearly anything. That is why, one electron present in the hydrogen atom can form a covalent bond with the unpaired electron of. 100% (1 rating) valence of hydrogen atom is 1. This is the reason why h is always a terminal atom and never a central atom. There are exactly the right numbers of + hydrogens.
How many hydrogen bonds are attached to each water molecule in a solid
Web since hydrogen has only one valence electron, it will only bond once. Thus one hydrogen atom will only bond once. 100% (1 rating) valence of hydrogen atom is 1. Which of the following species cannot exist? Web how many bonds can an atom form?
PPT hydrogen bond PowerPoint Presentation, free download ID4524678
Recognising where there are lone pairs that. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. There are exactly the right numbers of + hydrogens. 1 (apex) how many covalent bonds will a hydrogen atom normally make? That is why, one electron present in the hydrogen atom can form a covalent bond with.
PPT Chemistry of Life PowerPoint Presentation, free download ID2666943
Web hydrogen only needs to form one bond to complete a duet of electrons. 100% (1 rating) valence of hydrogen atom is 1. A hydrogen atom will typically form two. Web how many bonds can an atom form? There are exactly the right numbers of + hydrogens.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Web apart from some group 13 weirdness, hydrogen can only make one bond. Web how many bonds can a hydrogen atom form? Web cbse notes live join vedantu’s free mastercalss what is a hydrogen bond? Hydrogen bonds with hydrogen bond acceptor atoms such as oxygen. 100% (1 rating) valence of hydrogen atom is 1.
how many bonds does sulfur form
Web what type of bond can hydrogen atom form? 100% (1 rating) valence of hydrogen atom is 1. Hydrogen bonds with hydrogen bond acceptor atoms such as oxygen. Which of the following species cannot exist? Thus one hydrogen atom will only bond once.
Difference Between Intermolecular and Intramolecular Hydrogen Bonding
Hydrogen bonds with hydrogen bond acceptor atoms such as oxygen. A hydrogen atom will typically form two. Which of the following species cannot exist? 100% (1 rating) valence of hydrogen atom is 1. Thus one hydrogen atom will only bond once.
Properties of Water Presentation Biology
Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Recognising where there are lone pairs that. This is the reason why h is always a terminal atom and never a central atom. Web for example, in methane (ch _4 4 ), carbon forms covalent bonds with four hydrogen atoms. That is why, one.
HONC 1234 ChemSimplified
Each bond corresponds to a pair of shared electrons (one from carbon and one. Thus one hydrogen atom will only bond once. Web cbse notes live join vedantu’s free mastercalss what is a hydrogen bond? 100% (1 rating) valence of hydrogen atom is 1. Web what type of bond can hydrogen atom form?
A Hydrogen Atom Will Typically Form Two.
This is the reason why h is always a terminal atom and never a central atom. There are exactly the right numbers of + hydrogens. Hydrogen bonds with hydrogen bond acceptor atoms such as oxygen. Web apart from some group 13 weirdness, hydrogen can only make one bond.
Covalent Bonds With Nearly Anything.
100% (1 rating) valence of hydrogen atom is 1. Recognising where there are lone pairs that. Web what type of bond can hydrogen atom form? Each bond corresponds to a pair of shared electrons (one from carbon and one.
Web How Many Bonds Can An Atom Form?
Which of the following species cannot exist? A hydrogen bond is a kind of bonding that is present between an atom of. Web how many bonds can a hydrogen atom form? That is why, one electron present in the hydrogen atom can form a covalent bond with the unpaired electron of.
Web Cbse Notes Live Join Vedantu’s Free Mastercalss What Is A Hydrogen Bond?
Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Covalent bonds require pairs of electrons and hydrogen can only have two electrons bound in one. 1 (apex) how many covalent bonds will a hydrogen atom normally make? Web hydrogen only needs to form one bond to complete a duet of electrons.




.PNG)
