How Long Does It Take For Rust To Form
How Long Does It Take For Rust To Form - Rust chemistry is fairly straightforward: The only metals that rust are steel and iron. Web by julie richards. Certain environmental factors, such as salt water and acid rain, accelerate the rusting process. However, in some cases, it can take several months or even years for rust to form, especially if the metal is well protected and kept in a dry environment. For starters, different grades of steel rust at different speeds. Web rust takes four to five days to form on a car after the paint has been damaged. Other metals may become corroded but they do not rust. 1) the iron can have additives to prevent rusting. Rust is a naturally occurring phenomenon when certain metals are exposed to oxygen and water for a length of time.
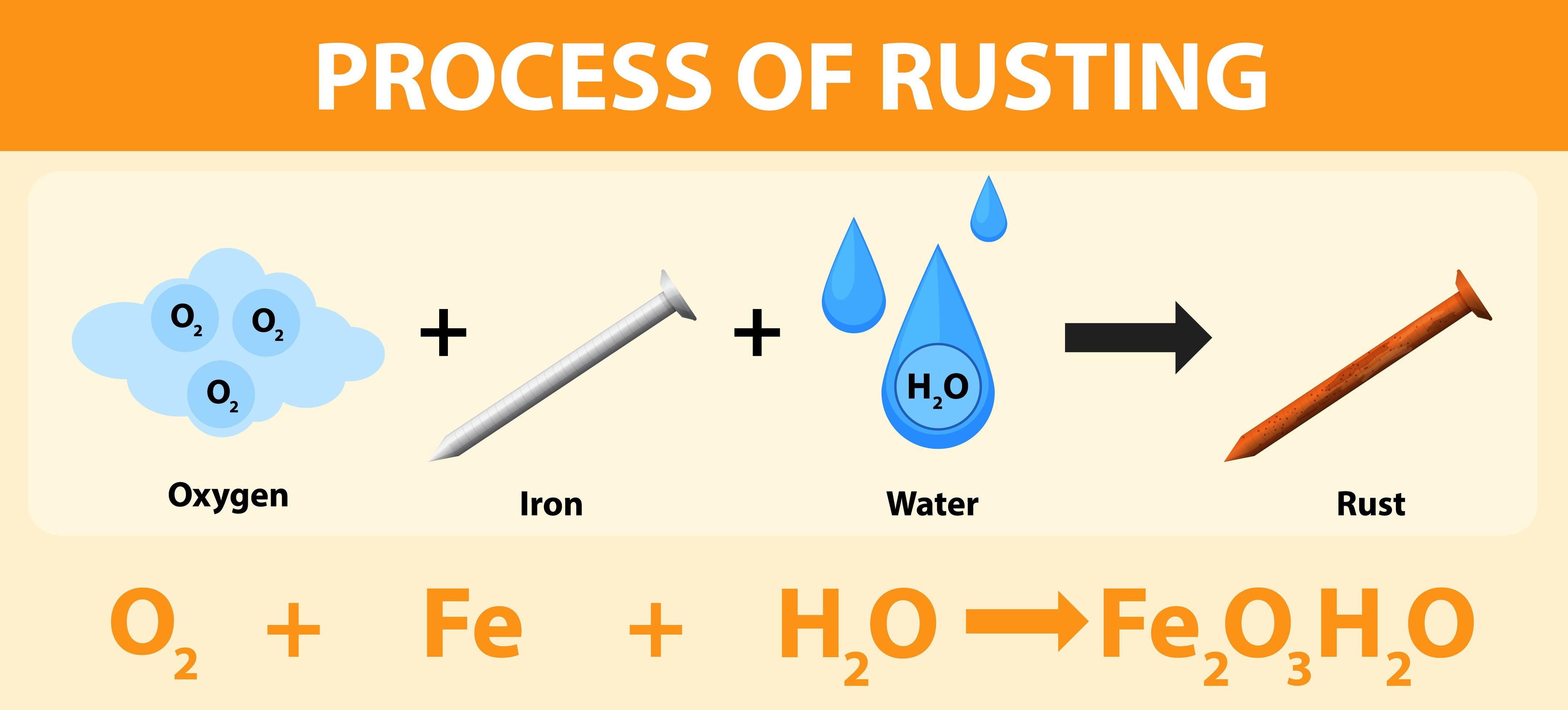
Rust requires three chemicals in order to form: My impression is that the rust web ecosystem priorities performance and expressiveness over simplicity and understandability, so getting in that particular area is hard. Other metals may become corroded but they do not rust. Rust chemistry is fairly straightforward: Web by julie richards. Certain environmental factors, such as salt water and acid rain, accelerate the rusting process. Of course, there are plenty of factors that have the potential to skew the rate of rust formation. The filings in jar 1 and jar 4 will show rust; Web rusting can happen quickly or slowly, depending on the material that’s rusting, and the environment. The only metals that rust are steel and iron.
Web it will also depend to some extent on the precise makeup of the metal which is being rusted or corroded. When rusting occurs, iron atoms lose electrons to the oxygen atoms. Rust chemistry is fairly straightforward: If all the accelerating features are present at one time, and if the metal being operated on is thin, it can be eaten through in a very short period of time, generally a matter of weeks or months. It occurs in moist air and in water. For this process to happen three things need to be present, an anode, a cathode and an electrolyte. Web by julie richards. Rust is the oxidation of iron along with the absorption of water to make fe2o3 with water molecules attached. My impression is that the rust web ecosystem priorities performance and expressiveness over simplicity and understandability, so getting in that particular area is hard. Jar 4 is likely to have more rust than jar 1.
What Is Rust? ERPS
1) the iron can have additives to prevent rusting. The filings in jar 2 and 3 will not. Other metals may become corroded but they do not rust. Web rust is iron oxide, or a molecule that has two iron atoms and three oxygen atoms. Of course, there are plenty of factors that have the potential to skew the rate.
How Long Does Corten Steel Take to Rust?
Certain environmental factors, such as salt water and acid rain, accelerate the rusting process. The filings in jar 1 and jar 4 will show rust; Web it will also depend to some extent on the precise makeup of the metal which is being rusted or corroded. Web by julie richards. The filings in jar 2 and 3 will not.
How Long Does It Take for Rust to Form? AZ Rust
If all the accelerating features are present at one time, and if the metal being operated on is thin, it can be eaten through in a very short period of time, generally a matter of weeks or months. It occurs in moist air and in water. When rusting occurs, iron atoms lose electrons to the oxygen atoms. Rust is a.
Process of rusting chemical equation 1868434 Vector Art at Vecteezy
Of course, there are plenty of factors that have the potential to skew the rate of rust formation. Certain environmental factors, such as salt water and acid rain, accelerate the rusting process. When oxygen and water come in contact with the metal, they cause a chemical reaction that creates iron oxide. Although about 21% of air consists of oxygen, .
How Does Rust Spread? Sciencing
My impression is that the rust web ecosystem priorities performance and expressiveness over simplicity and understandability, so getting in that particular area is hard. However, in some cases, it can take several months or even years for rust to form, especially if the metal is well protected and kept in a dry environment. Web rust takes four to five days.
How Long Does It Take Metal to Rust? 3 Clear Answers
Rust is the oxidation of iron along with the absorption of water to make fe2o3 with water molecules attached. Web rust takes four to five days to form on a car after the paint has been damaged. Other metals may become corroded but they do not rust. The filings in jar 1 and jar 4 will show rust; Web rust.
How Long Does It Take to Form A Habit?
The only metals that rust are steel and iron. Web rusting can happen quickly or slowly, depending on the material that’s rusting, and the environment. Web rust forms when oxygen reacts with iron, but simply putting iron and oxygen together isn't sufficient. It is the result of an electrochemical process that we know more commonly as corrosion. The filings in.
How To Rust Day 93! How Long Will My Pickaxe Raid Take? YouTube
Rust chemistry is fairly straightforward: Certain environmental factors, such as salt water and acid rain, accelerate the rusting process. Web by julie richards. Web rusting can happen quickly or slowly, depending on the material that’s rusting, and the environment. Rust requires three chemicals in order to form:
Does Engine Oil Prevent Rust? Oil Genesis
However, in some cases, it can take several months or even years for rust to form, especially if the metal is well protected and kept in a dry environment. When rusting occurs, iron atoms lose electrons to the oxygen atoms. The filings in jar 1 and jar 4 will show rust; When oxygen and water come in contact with the.
How Does Rust Form? Sciencing
Certain environmental factors, such as salt water and acid rain, accelerate the rusting process. Rust is a naturally occurring phenomenon when certain metals are exposed to oxygen and water for a length of time. For this process to happen three things need to be present, an anode, a cathode and an electrolyte. Web rust is iron oxide, or a molecule.
When Oxygen And Water Come In Contact With The Metal, They Cause A Chemical Reaction That Creates Iron Oxide.
So how does rust form, exactly? Web rust takes four to five days to form on a car after the paint has been damaged. However, in some cases, it can take several months or even years for rust to form, especially if the metal is well protected and kept in a dry environment. Rust is the oxidation of iron along with the absorption of water to make fe2o3 with water molecules attached.
The Filings In Jar 2 And 3 Will Not.
Web by julie richards. The only metals that rust are steel and iron. Web rusting can happen quickly or slowly, depending on the material that’s rusting, and the environment. For starters, different grades of steel rust at different speeds.
Certain Environmental Factors, Such As Salt Water And Acid Rain, Accelerate The Rusting Process.
Rust requires three chemicals in order to form: For this process to happen three things need to be present, an anode, a cathode and an electrolyte. Here are some things that can affect the rusting rate: Rust is a naturally occurring phenomenon when certain metals are exposed to oxygen and water for a length of time.
When Rusting Occurs, Iron Atoms Lose Electrons To The Oxygen Atoms.
Jar 4 is likely to have more rust than jar 1. It is the result of an electrochemical process that we know more commonly as corrosion. It occurs in moist air and in water. Although about 21% of air consists of oxygen, rusting doesn't occur in dry air.