How Do Nonmetals Form Negative Ions
How Do Nonmetals Form Negative Ions - Web this is actually one of the chemical properties of metals and nonmetals: By losing one or more electrons. Metals tend to form cations, while nonmetals tend to form anions. By bonding with other negative ions. By losing one or more. To obtain a full outer shell: Web ions form when atoms lose or gain electrons. See answer (1) best answer. These are electronegative elements with high ionization. Web they gain electrons to become negative or an ion.
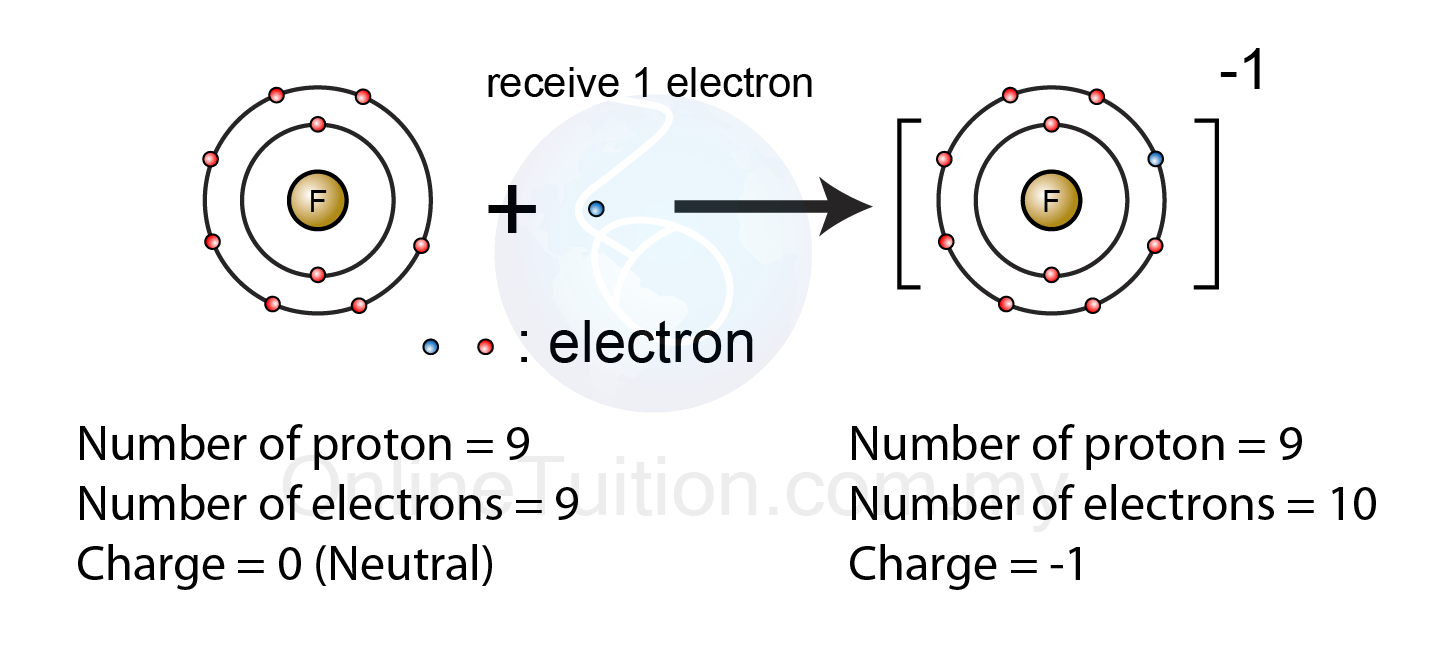
Web ions form when atoms lose or gain electrons. See answer (1) best answer. Web this is actually one of the chemical properties of metals and nonmetals: Metals tend to form cations, while nonmetals tend to form anions. Negatively charged ions are called. Thus, the electron affinity will be. Web they gain electrons to become negative or an ion. By bonding with other negative ions. How do nonmetals form negative ions? Web when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons.
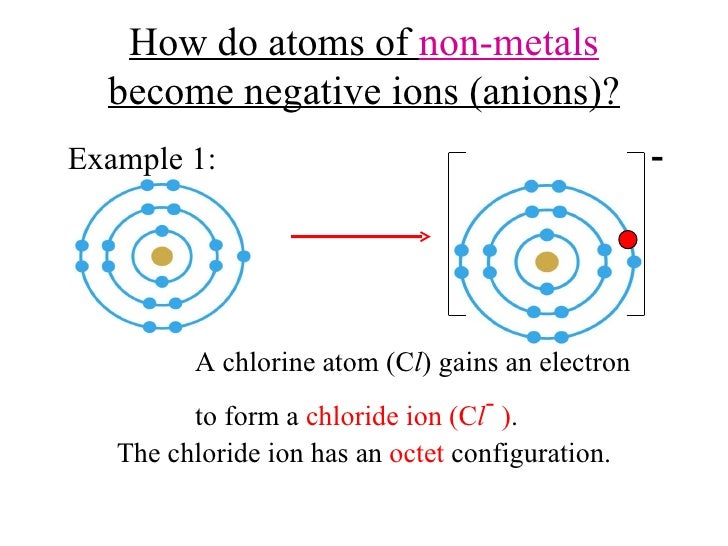
How do nonmetals form negative ions? By losing one or more. Web this is actually one of the chemical properties of metals and nonmetals: See answer (1) best answer. By bonding with other negative ions. Fx−, clx−, ix−, sx2− f x −, c l x −, i x −, s x 2 − multi. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Ions can be either monatomic (containing only. Thus, the electron affinity will be. Negatively charged ions are called.
Formation of Negative Ions SPM Chemistry
See answer (1) best answer. Thus, the electron affinity will be. Web they gain electrons to become negative or an ion. Web this is actually one of the chemical properties of metals and nonmetals: By bonding with other negative ions.
SOLVEDNonmetals form negative ions by (losing/gaining) enough
Web this is actually one of the chemical properties of metals and nonmetals: Negatively charged ions are called. Positively charged ions are called cations, and negatively charge ions are called anions. By bonding with other negative ions. See answer (1) best answer.
Ions Predict Charge Stone Cold Chemistry Talk Ions Predict Charge
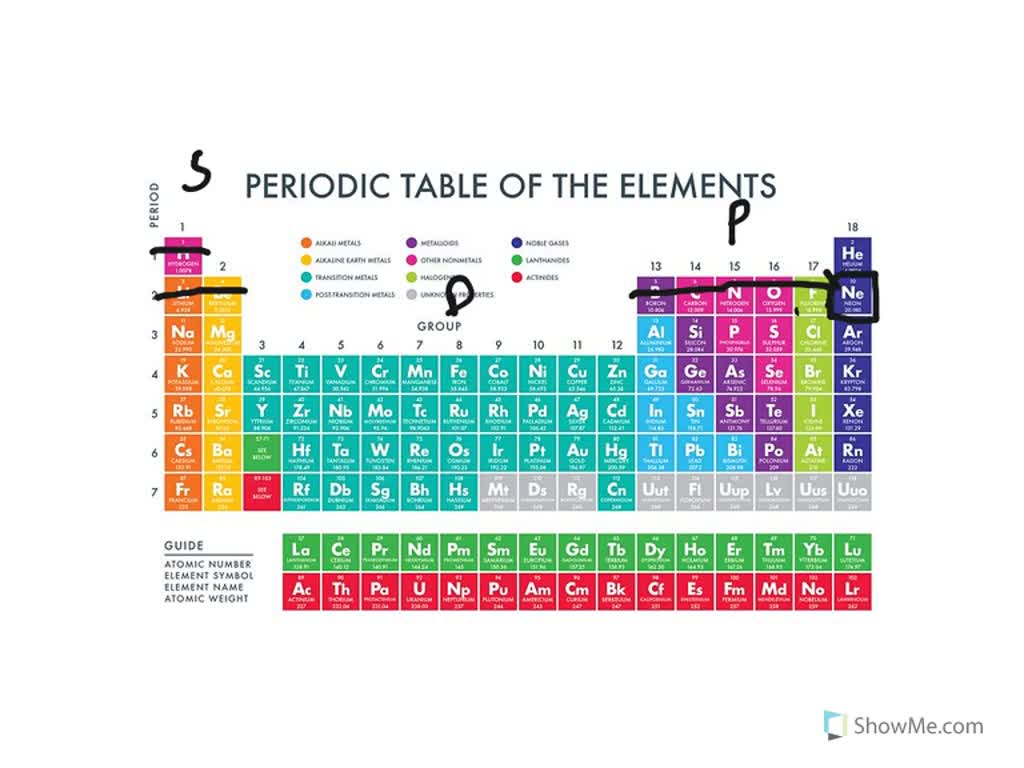
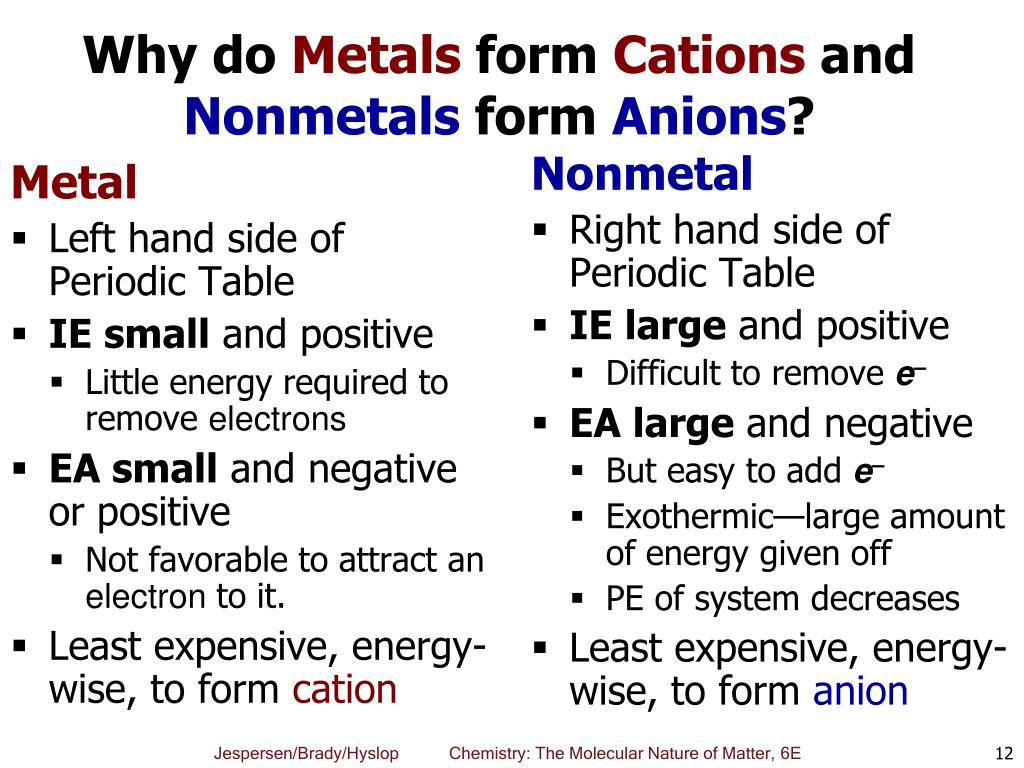
Negatively charged ions are called. Web glossary learning objectives define ionic compounds predict the type of compound formed from elements based on their location within the periodic table. Positively charged ions are called cations, and negatively charge ions are called anions. Metals tend to form cations, while nonmetals tend to form anions. By losing one or more electrons.
Hydrogen Valence Electrons In Hydrogen
Web when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Positively charged ions are called cations, and negatively charge ions are called anions. Fx−, clx−, ix−, sx2− f x −, c l x −, i x −, s x 2 − multi. These are electronegative elements with high ionization. Web glossary.
The Parts of the Periodic Table
By losing one or more electrons. How do nonmetals form ions? Web ions form when atoms lose or gain electrons. Web they gain electrons to become negative or an ion. Web when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons.
table of elements chart
Web when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Ions can be either monatomic (containing only. See answer (1) best answer. Thus, the electron affinity will be. These are electronegative elements with high ionization.
PPT Chapter 9 The Basics of Chemical Bonding PowerPoint Presentation
Negatively charged ions are called. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Web ions form when atoms lose or gain electrons. Web thus, nonmetals tend to form negative ions. By losing one or more.
Electron Affinity of The Elements
Negatively charged ions are called. Web this is actually one of the chemical properties of metals and nonmetals: Web when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. To obtain a full outer shell: By bonding with other negative ions.
Nonmetals and anion formation YouTube
Negatively charged ions are called. To obtain a full outer shell: The ions formed are negative, because they have more electrons than protons the ions have the. Web they gain electrons to become negative or an ion. Web when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons.
Chem matters ch6_ionic_bond
Metals tend to form cations, while nonmetals tend to form anions. By losing one or more. Fx−, clx−, ix−, sx2− f x −, c l x −, i x −, s x 2 − multi. How do nonmetals form negative ions? The ions formed are negative, because they have more electrons than protons the ions have the.
How Do Nonmetals Form Negative Ions?
Thus, the electron affinity will be. To obtain a full outer shell: Negatively charged ions are called. Web they gain electrons to become negative or an ion.
Positively Charged Ions Are Called Cations, And Negatively Charge Ions Are Called Anions.
Web when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Web this is actually one of the chemical properties of metals and nonmetals: By bonding with other negative ions. How do nonmetals form ions?
Web Thus, Nonmetals Tend To Form Negative Ions.
These are electronegative elements with high ionization. Web when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. By losing one or more. See answer (1) best answer.
The Ions Formed Are Negative, Because They Have More Electrons Than Protons The Ions Have The.
Web glossary learning objectives define ionic compounds predict the type of compound formed from elements based on their location within the periodic table. By losing one or more electrons. Ions can be either monatomic (containing only. Negatively charged ions are called.