Form Fda 3537
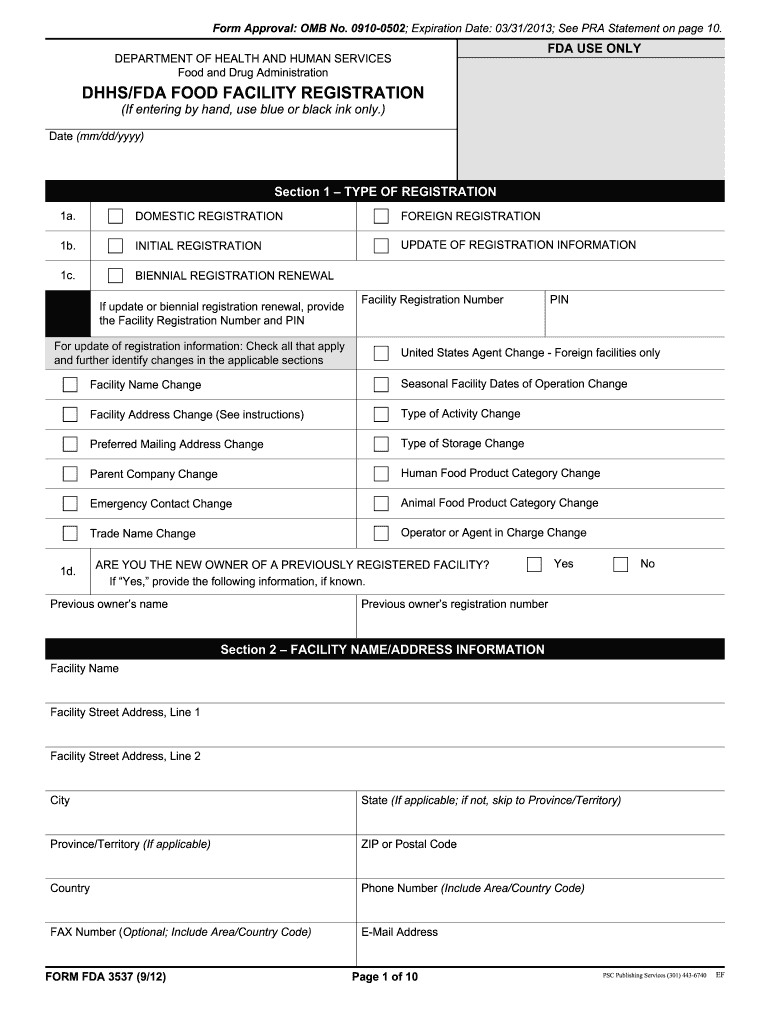
Form Fda 3537 - Web form fda 3537 (9/12) dhhs/fda food facility registration page 5 of 10 to be completed by all food facilities. This form is available online and in paper form. Easily fill out pdf blank, edit, and sign them. Upload, modify or create forms. You may obtain a copy of this form by writing to the u.s. Please see instructions for further examples. Food and drug administration, center for food safety and. Save or instantly send your ready documents. Form fda 3537 (8/11) page 1 of 6. Web dhhs/fda food facility registration.
If you have any questions before registering or you feel your facility may be exempt, please send to. Web dhhs/fda food facility registration. You may obtain a copy of this form by writing to the. Web use form 3537 to register, renew or update a registration. Web instructions for form 3537 food facility registration form note: Form fda 3537 (8/11) page 1 of 6. 1.233 are there optional items included in the registration form? Fda use only department of health and human. Food and drug administration, center for food safety and. Web to carry out certain provisions of the bioterrorism act, fda established regulations requiring that:
Web use form 3537 to register, renew or update a registration. Food facilities register with fda, and; Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Fda requires you to provide the following. Web to carry out certain provisions of the bioterrorism act, fda established regulations requiring that: Easily fill out pdf blank, edit, and sign them. Web form fda 3537 (9/12) dhhs/fda food facility registration page 5 of 10 to be completed by all food facilities. You may obtain a copy of this form by writing to the. Web instructions for form 3537 food facility registration form note: Use the following instructions to download the form if.
Form FDA 3537 Fill Out and Sign Printable PDF Template signNow
Addition of unique facility identifier (ufi) field in section 2. Save or instantly send your ready documents. You may obtain a copy of this form by writing to the u.s. Easily fill out pdf blank, edit, and sign them. Abbreviated registration renewal (complete section 12) by checking this.
Form FDA 3537a DHHS/FDA Cancellation of Food Facility Registration
Easily fill out pdf blank, edit, and sign them. Fda encourages, but does not require, you to submit items that are indicated as optional. Form 3537 is used to register a food facility or to provide an update to an existing registration. Web instructions for form 3537 food facility registration form note: Please see instructions for further examples.
A Look at the FDA Registration Renewal
Ad form fda 3537 & more fillable forms, register and subscribe now! Web (1) you must register or renew a registration (including abbreviated registration renewals) using form fda 3537. Web (1) you must update your registration using form fda 3537. Create a new form 3537 please read and understand the information below. Abbreviated registration renewal (complete section 12) by checking.
Form FDA 3627 A Guide for the Submission of Initial Reports Free Download
You may obtain a copy of this form by writing to the u.s. Web (1) you must update your registration using form fda 3537. Addition of unique facility identifier (ufi) field in section 2. If you have any questions before registering or you feel your facility may be exempt, please send to. Upload, modify or create forms.
Form FDA 2512a Cosmetic Product Ingredient Statement [CFSAN] Free
Upload, modify or create forms. Web up to $40 cash back form approval omb no. Web (1) you must update your registration using form fda 3537. Ad form fda 3537 & more fillable forms, register and subscribe now! Easily fill out pdf blank, edit, and sign them.
Form FDA 2511 Registration of Cosmetic Product Establishment Free
Web to carry out certain provisions of the bioterrorism act, fda established regulations requiring that: Abbreviated registration renewal (complete section 12) by checking this. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Web form fda 3537 (9/12) dhhs/fda food facility registration page 5 of 10 to be completed by.
FDA3537_508 (9.12) Zip Code Grain Free 30day Trial Scribd
Food and drug administration, center for food safety and. 1.233 are there optional items included in the registration form? You may obtain a copy of this form by writing to the u.s. If you have any questions before registering or you feel your facility may be exempt, please send to. Web to carry out certain provisions of the bioterrorism act,.
Form FDA 3613c Supplementary Information NonClinical Research Use
Easily fill out pdf blank, edit, and sign them. If you have any questions before registering or you feel your facility may be exempt, please send to. Form 3537 is used to register a food facility or to provide an update to an existing registration. Web up to $40 cash back form approval omb no. Ad form fda 3537 &.
Form FDA 2767 Notice of Availability of Sample Electronic Product
Easily fill out pdf blank, edit, and sign them. Food and drug administration, center for food safety and. Easily fill out pdf blank, edit, and sign them. This form is available online and in paper form. Web up to $40 cash back form approval omb no.
Form FDA 3630 Guide for Preparing Product Reports on Sunlamps Free
Try it for free now! Food and drug administration, center for food safety and. Web to carry out certain provisions of the bioterrorism act, fda established regulations requiring that: You may obtain a copy of this form by writing to the u.s. Department of health and human services food and drug administration.
Web To Carry Out Certain Provisions Of The Bioterrorism Act, Fda Established Regulations Requiring That:
Ad form fda 3537 & more fillable forms, register and subscribe now! Fda requires you to provide the following. If you have any questions before registering or you feel your facility may be exempt, please send to. While the fda expects all registrants to provide their duns number.
Web Dhhs/Fda Food Facility Registration.
Addition of unique facility identifier (ufi) field in section 2. Form fda 3537 (8/11) page 1 of 6. Fda encourages, but does not require, you to submit items that are indicated as optional. Easily fill out pdf blank, edit, and sign them.
1.233 Are There Optional Items Included In The Registration Form?
Web form fda 3537 (9/12) dhhs/fda food facility registration page 5 of 10 to be completed by all food facilities. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Web complete form fda 3537 online with us legal forms. Easily fill out pdf blank, edit, and sign them.
This Form Is Available Online And In Paper Form.
Food and drug administration, center for food safety and. Web up to $40 cash back form approval omb no. Web use form 3537 to register, renew or update a registration. You may obtain a copy of this form by writing to the.



![Form FDA 2512a Cosmetic Product Ingredient Statement [CFSAN] Free](https://www.formsbirds.com/formimg/additional-fda-forms/17723/form-fda-2512a-cosmetic-product-ingredient-statement-cfsan-l2.png)




