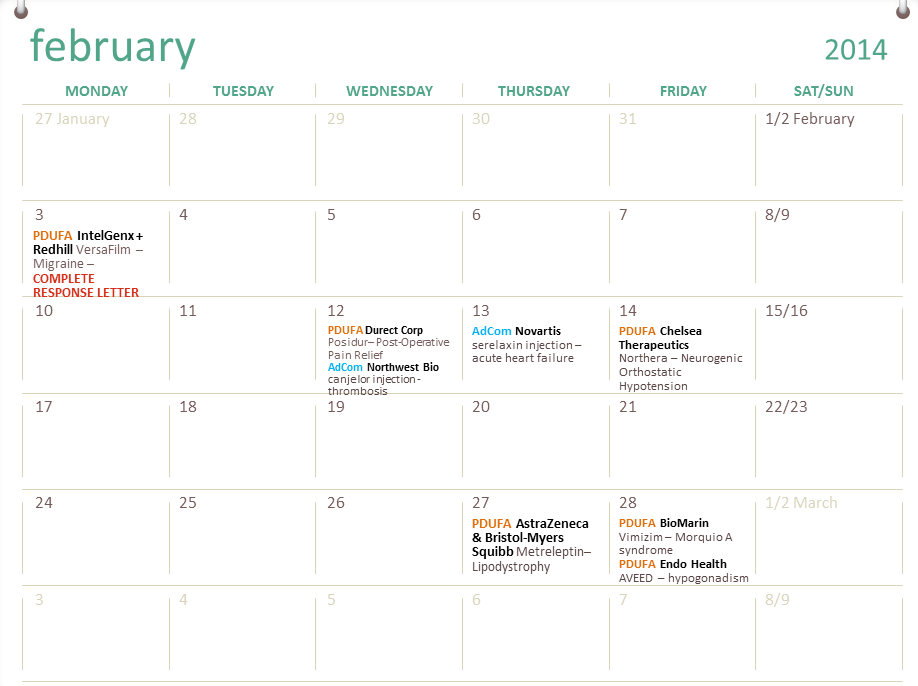
Fda Calendar For Drug Approval
Fda Calendar For Drug Approval - Innovative drugs often mean new treatment options for patients. Resources for information | approved drugs. Web cder drug and biologic approvals for calendar year 2022; Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching,. Cder drug and biologic approvals for calendar year. Cder highlights key web sites. Web this week's drug approvals. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital.
Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for. Cder highlights key web sites. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Resources for information | approved drugs. Web cder drug and biologic approvals for calendar year 2022; Web this week's drug approvals. Innovative drugs often mean new treatment options for patients. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching,. Web 24 rows novel drug approvals for 2023.
Innovative drugs often mean new treatment options for patients. Cder highlights key web sites. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching,. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Web cder drug and biologic approvals for calendar year 2022; Cder drug and biologic approvals for calendar year. Resources for information | approved drugs. Web 24 rows novel drug approvals for 2023. Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for.
FDA Calendar FDA Tracker
Resources for information | approved drugs. Cder drug and biologic approvals for calendar year. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching,. Cder highlights key web sites. Web this week's drug approvals.
40 Great Medication Schedule Templates (+Medication Calendars)
Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Cder drug and biologic approvals for calendar year. Innovative drugs often mean new treatment options for patients. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching,. Web 24 rows novel drug approvals for 2023.
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Resources for information | approved drugs. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Human drugs and.
FDA Calendar FDA Tracker
Innovative drugs often mean new treatment options for patients. Cder highlights key web sites. Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Web this week's drug approvals. Resources for information | approved drugs.
FDA Calendar FDA Tracker
Cder drug and biologic approvals for calendar year. Web cder drug and biologic approvals for calendar year 2022; Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for. Innovative drugs often mean new treatment options for patients. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching,.
UPDATED The Global Pharmaceutical Serialisation Calendar 2014 2018
Innovative drugs often mean new treatment options for patients. Web 24 rows novel drug approvals for 2023. Resources for information | approved drugs. Web cder drug and biologic approvals for calendar year 2022; Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in.
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
Web cder drug and biologic approvals for calendar year 2022; Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Cder drug and biologic approvals for calendar year. Resources for information | approved drugs. Innovative drugs often mean new treatment options for patients.
The Most Important New Drug Of 2014
Cder highlights key web sites. Resources for information | approved drugs. Cder drug and biologic approvals for calendar year. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital.
FDA Calendar FDA Tracker
Web cder drug and biologic approvals for calendar year 2022; Cder highlights key web sites. Innovative drugs often mean new treatment options for patients. Cder drug and biologic approvals for calendar year. Resources for information | approved drugs.
FDA Calendar FDA Tracker
Web 24 rows novel drug approvals for 2023. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Innovative drugs often mean new treatment options for patients. Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Web the fda’s 2024 pdufa calendar is far from complete,.
Web This Week's Drug Approvals.
Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching,. Cder highlights key web sites. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in.
Innovative Drugs Often Mean New Treatment Options For Patients.
Cder drug and biologic approvals for calendar year. Web cder drug and biologic approvals for calendar year 2022; Web 24 rows novel drug approvals for 2023. Web an fda calendar typically displays information about the expected timeline for a particular drug approval or.
Human Drugs And Therapeutic Biologicals (Proteins And Other Products Derived From Living Sources Used For.
Resources for information | approved drugs.