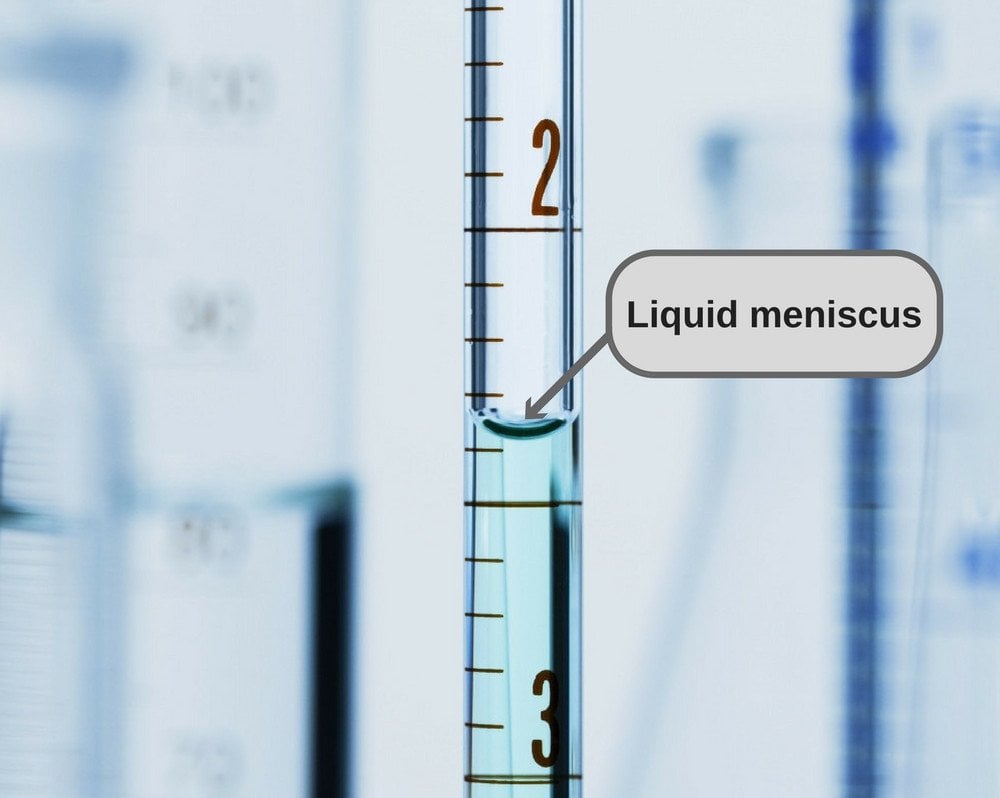
Explain Why Liquids In A Test Tube Form A Meniscus
Explain Why Liquids In A Test Tube Form A Meniscus - Web menisci are a manifestation of capillary action, by which either surface adhesion pulls a liquid up to form a concave meniscus, or internal cohesion pulls the liquid down to. Web learn about the meniscus of liquids as well as convex and concave meniscus. Where it meets the wall of the container is obscured. Red and blue marbles of the same size and mass chemistry Tubes made out of material that water does not stick to. Most liquids have an attraction between their molecules that is stronger than the. Adhesion to the test tube or cohesion of the liquid molecules causes the meniscus to form. Web a flat meniscus occurs with water in some types of plastic tubes; Web the meniscus is the curvature of the fluid surface. Web when water is confined in a glass tube, its meniscus (surface) has a concave shape because the water wets the glass and creeps up the side of the tube.
Red and blue marbles of the same size and mass chemistry When you put water in a beaker or test tube, you see a curved surface. Web learn about the meniscus of liquids as well as convex and concave meniscus. Web menisci are a manifestation of capillary action, by which either surface adhesion pulls a liquid up to form a concave meniscus, or internal cohesion pulls the liquid down to. Tubes made out of material that water does not stick to. Web why do liquids in a test tube form a meniscus? A convex meniscus (sometimes called a backwards. The attraction is strong between the liquid molecule. Web the meniscus is the curvature of the fluid surface. | physics van | uiuc.
Surface tension and adhesive forces cause the formation of a meniscus. A convex meniscus (sometimes called a backwards. Web fluid molecules are attracted to the molecules in the wall of the glass beaker and since fluid molecules like to stick together, when the molecules touching the fluid cling to it, other. Web a meniscus is what happens when you put a liquid into a container. Web capillary action is responsible for the concave liquid surface, called a meniscus, that forms in a test tube or graduated cylinder. Web what is meniscus in test tube? Web why do liquids in a test tube form a meniscus? What is the wavelength of the electromagnetic wave used by this phone? A non dissolving solid mixed with a liquid c. Web a flat meniscus occurs when water in some types of plastic tubes;
Chapter 11.3 Unique Properties of Liquids Chemwiki
Red and blue marbles of the same size and mass chemistry Most liquids have an attraction between their molecules that is stronger than the. First, it is easiest to determine the bottom of the meniscus as it is very well defined. When you put water in a beaker or test tube, you see a curved surface. Discover why meniscus is.
Colored Liquids in Test Tubes Stock Image Image of equipment
Web a meniscus is what happens when you put a liquid into a container. The attraction is strong between the liquid molecule. Web a flat meniscus occurs with water in some types of plastic tubes; Web the liquid appears to stick to the edge of the container. Tubes made out of material that water does not stick to.
Warum bleibt Wasser beim Gießen am Glas haften? TUNLOG
Therefore, liquids in a test tube form a meniscus because the liquid. It occurs when the liquid is placed in the test tube due to the bonding force between the water molecules and the tank. When liquid is placed in a container, a meniscus forms. The key to getting an accurate reading is to measure the center of the meniscus.
Test tube meniscus Stock Image C009/7244 Science Photo Library
First, it is easiest to determine the bottom of the meniscus as it is very well defined. In any case, you get the true volume of the liquid. Web a flat meniscus occurs with water in some types of plastic tubes; Web what is meniscus in test tube? When liquid is placed in a container, a meniscus forms.
Why Can Liquids Flow But Solids Cannot
Web why do liquids in a test tube form a meniscus? Web fluid molecules are attracted to the molecules in the wall of the glass beaker and since fluid molecules like to stick together, when the molecules touching the fluid cling to it, other. Web a flat meniscus occurs when water in some types of plastic tubes; Tubes made out.
Surface Tension Chemwiki
In any case, you get the true volume of the liquid. Surface tension and adhesive forces cause the formation of a meniscus. Web why does water curve, and what is a meniscus? When you put water in a beaker or test tube, you see a curved surface. With most liquids, the attractive force.
fluid dynamics What properties of liquids determine the shape of the
The grainger college of engineering. Web what is meniscus in test tube? Surface tension of liquids.the tube forms a concave meniscus, which is a virtually spherical surface having the same radius, r , as the inside of the tube. Red and blue marbles of the same size and mass chemistry Web when water is confined in a glass tube, its.
Wallpaper liquids, colored liquids, test tubes images for desktop
Web pick up the glassware to bring it up to eye level or bend down to take a measurement. Web what is meniscus in test tube? Web the liquid appears to stick to the edge of the container. Web the meniscus is the curvature of the fluid surface. A convex meniscus (sometimes called a backwards.
chemistry titrations University of Birmingham
Therefore, liquids in a test tube form a meniscus because the liquid. Web a flat meniscus occurs when water in some types of plastic tubes; Web pick up the glassware to bring it up to eye level or bend down to take a measurement. Tubes made out of material that water does not stick to. The attraction is strong between.
3 BEST SelfTests for Torn Meniscus & 2 Tests to AVOID
The key to getting an accurate reading is to measure the center of the meniscus whether it. Where it meets the wall of the container is obscured. The attraction is strong between the liquid molecule. Web what is meniscus in test tube? It occurs when the liquid is placed in the test tube due to the bonding force between the.
Web Capillary Action Is Responsible For The Concave Liquid Surface, Called A Meniscus, That Forms In A Test Tube Or Graduated Cylinder.
Web a flat meniscus occurs with water in some types of plastic tubes; Web learn about the meniscus of liquids as well as convex and concave meniscus. Red and blue marbles of the same size and mass chemistry Discover why meniscus is important to liquids in science and how to read a meniscus.
A Non Dissolving Solid Mixed With A Liquid C.
A convex meniscus (sometimes called a backwards. Web a flat meniscus occurs when water in some types of plastic tubes; The grainger college of engineering. Web the liquid appears to stick to the edge of the container.
First, It Is Easiest To Determine The Bottom Of The Meniscus As It Is Very Well Defined.
Web menisci are a manifestation of capillary action, by which either surface adhesion pulls a liquid up to form a concave meniscus, or internal cohesion pulls the liquid down to. Web fluid molecules are attracted to the molecules in the wall of the glass beaker and since fluid molecules like to stick together, when the molecules touching the fluid cling to it, other. Web a meniscus is what happens when you put a liquid into a container. There are probably two reasons.
Surface Tension And Adhesive Forces Cause The Formation Of A Meniscus.
Surface tension of liquids.the tube forms a concave meniscus, which is a virtually spherical surface having the same radius, r , as the inside of the tube. It occurs when the liquid is placed in the test tube due to the bonding force between the water molecules and the tank. Most liquids have an attraction between their molecules that is stronger than the. Adhesion to the test tube or cohesion of the liquid molecules causes the meniscus to form.