Do Nonmetals Form Anions Or Cations
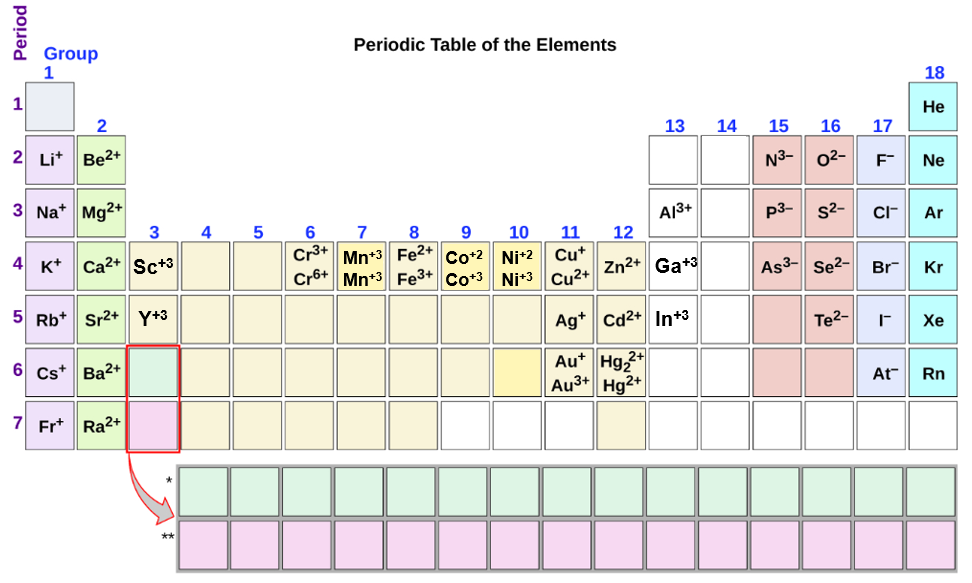
Do Nonmetals Form Anions Or Cations - A nitrogen atom must gain three electrons to have the same number of electrons as an atom of the following noble gas,. Ion has a different number of electrons. Anions are atoms that have a negative charge because they have gained one or more electrons. These are electronegative elements with high ionization energies. Do nonmetals form anions or cations? Metals tend to form cations, while nonmetals tend to form anions. Web solved:explain why metals tend to form cations, while nonmetals tend to form anions. Web nonmetals form negatively charged ions, or anions. Web this is actually one of the chemical properties of metals and nonmetals: Web most other metals form cations (e.g.
Anions are negative ions that are formed when a nonmetal atom gains one or more electrons. This occurs normally and usually naturally. Second, elements that live in the first two. Web this is actually one of the chemical properties of metals and nonmetals: Web do nonmetals form anions or cations? Metals tend to form cations, while nonmetals tend to form anions. Do nonmetals form anions or cations? There are cases where nonmetals can become cations. A nitrogen atom must gain three electrons to have the same number of electrons as an atom of the following noble gas,. Anions are atoms that have a negative charge because they have gained one or more electrons.
Web terms in this set (60) what distinguishes a neutral atom from an ion. Anions are negative ions that are formed when a nonmetal atom gains one or more electrons. Do nonmetals form anions or cations? Second, elements that live in the first two. Anions are atoms that have a negative charge because they have gained one or more electrons. Identify the reason why metals tend to form cations and nonmetals tend to form anions when these elements exist in a compound. Web this is actually one of the chemical properties of metals and nonmetals: They do this because they need to gain one to three electrons in order to achieve an octet of valence electrons,. Web most other metals form cations (e.g. Web non metals tend to form anions as they gain electron (s).
4.3 Ionic Compounds and Formulas (2022)
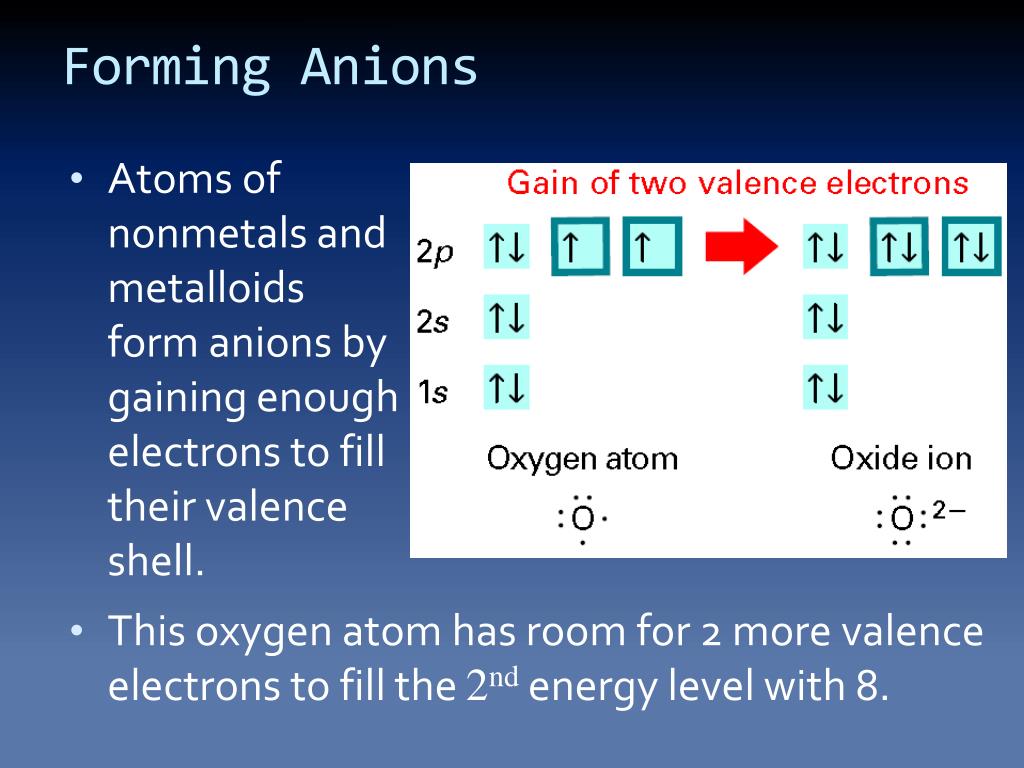
Web this is actually one of the chemical properties of metals and nonmetals: Anions are atoms that have a negative charge because they have gained one or more electrons. Second, elements that live in the first two. They do this because they need to gain one to three electrons in order to achieve an octet of valence electrons,. These are.
PPT IONS PowerPoint Presentation, free download ID2435906
Metals tend to form cations, while nonmetals tend to form anions. Web do nonmetals form anions or cations? Anions are atoms that have a negative charge because they have gained one or more electrons. Web terms in this set (60) what distinguishes a neutral atom from an ion. Anions are so named because they are attracted to the.
anion Common anions, their names, formulas and the elements they are
Anions are so named because they are attracted to the. Web this is actually one of the chemical properties of metals and nonmetals: Web answer (1 of 3): Web nonmetals form negatively charged ions, or anions. These are electronegative elements with high ionization energies.
Ions Predict Charge Stone Cold Chemistry Talk Ions Predict Charge
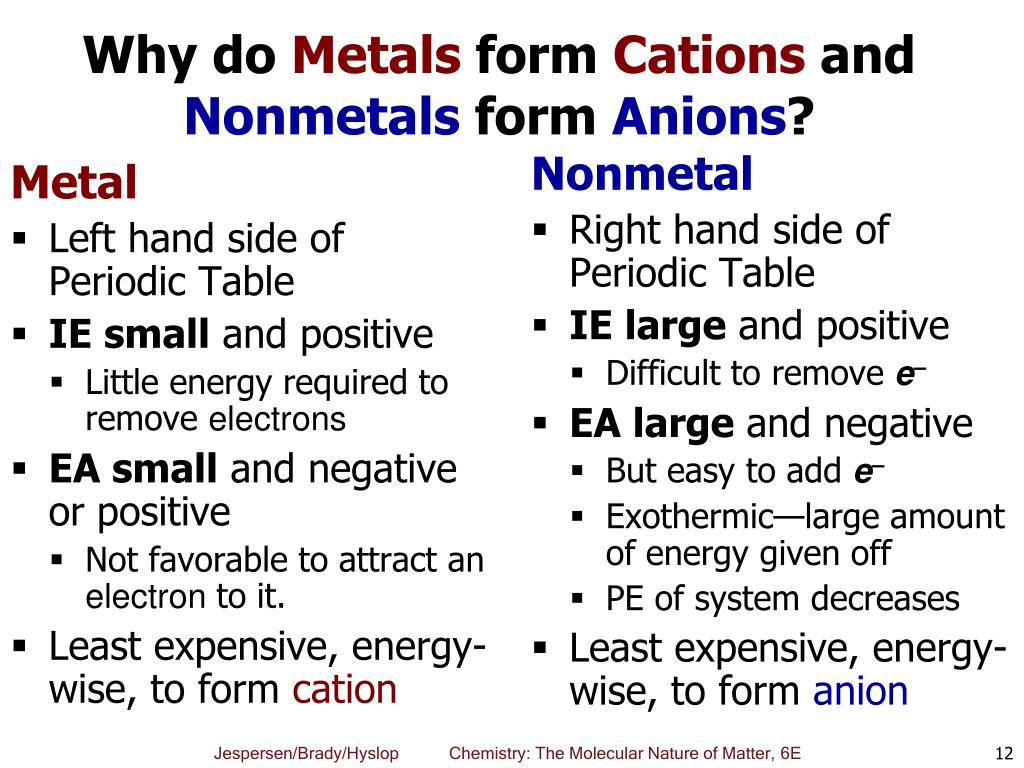
1 metals have low lonization energies. Web solved:explain why metals tend to form cations, while nonmetals tend to form anions. Web answer (1 of 3): Web nonmetals form negative ions (anions). Web this is actually one of the chemical properties of metals and nonmetals:
is incorreer? all s area eleseis erand e are metals b, all p area
These are electronegative elements with high ionization energies. Anions are atoms that have a negative charge because they have gained one or more electrons. There are cases where nonmetals can become cations. Web do nonmetals form anions or cations? Web answer (1 of 3):
Do metals form anions or cations quizlet? Book Vea
Ion has a different number of electrons. Web answer (1 of 3): Web nonmetals form negative ions (anions). Web non metals tend to form anions as they gain electron (s). Identify the reason why metals tend to form cations and nonmetals tend to form anions when these elements exist in a compound.
Cute cartoon the Elements Educational of Periodic Table Fabric poster
These are electronegative elements with high ionization energies. Web do nonmetals form anions or cations? A nitrogen atom must gain three electrons to have the same number of electrons as an atom of the following noble gas,. Web nonmetals form negatively charged ions, or anions. This occurs normally and usually naturally.
Nonmetals and anion formation YouTube
Web most other metals form cations (e.g. 1 metals have low lonization energies. Metals tend to form cations, while nonmetals tend to form anions. Anions are so named because they are attracted to the. Web answer (1 of 3):
PPT Chapter 9 The Basics of Chemical Bonding PowerPoint Presentation
Anions are atoms that have a negative charge because they have gained one or more electrons. Metals tend to form cations, while nonmetals tend to form anions. Anions are so named because they are attracted to the. 1 metals have low lonization energies. Web do nonmetals form anions or cations?
Difference Between Metals Nonmetals and Metalloids Definition
Web non metals tend to form anions as they gain electron (s). Web nonmetals form negatively charged ions, or anions. Web most other metals form cations (e.g. Anions are negative ions that are formed when a nonmetal atom gains one or more electrons. Ion has a different number of electrons.
Web Do Nonmetals Form Anions Or Cations?
Identify the reason why metals tend to form cations and nonmetals tend to form anions when these elements exist in a compound. Web non metals tend to form anions as they gain electron (s). Web this is actually one of the chemical properties of metals and nonmetals: They do this because they need to gain one to three electrons in order to achieve an octet of valence electrons,.
Web Answer (1 Of 3):
Metals tend to form cations, while nonmetals tend to form anions. Anions are negative ions that are formed when a nonmetal atom gains one or more electrons. Web this is actually one of the chemical properties of metals and nonmetals: Do nonmetals form anions or cations?
This Occurs Normally And Usually Naturally.
Second, elements that live in the first two. Iron, silver, nickel), whilst most other nonmetals typically form anions (e.g. Anions are atoms that have a negative charge because they have gained one or more electrons. Holt chemistry r.thomas myers, keith oldham,savatore tocci 2006 edition chapter 5,.
Web Nonmetals Form Negatively Charged Ions, Or Anions.
Web terms in this set (60) what distinguishes a neutral atom from an ion. Web most other metals form cations (e.g. Ion has a different number of electrons. Web nonmetals form negative ions (anions).