Chemistry Chapter 7
Chemistry Chapter 7 - Atoms of which elements (metals or nonmetals) tend to lose electrons? Web the gram atomic mass (gam), gram molecular mass (gmm), and gram formula mass (gfm) are the mass of one mole of an element, a molecular compound, and an ionic compound, respectively. Elements in human body (in order of proportions from greatest to least) oxygen, carbon, hydrogen, nitrogen, calcium. Positive charges form when electrons are lost. The elements are organized by. +91 8800440559 | +91 8448440632 The protons in the nucleus do not change during normal chemical reactions. Measurements and analysis in the atmosphere. Web unit 7 electronic structure of atoms unit 8 periodic table unit 9 chemical bonds unit 10 gases and kinetic molecular theory unit 11 states of matter and intermolecular forces unit 12 chemical equilibrium unit 13 acids. Limiting reactant click the card to flip 👆 a.
Measurements and analysis in the atmosphere. Something scientists use to represent an object or an event in order to make it. The forming of fog in the morning. Quickly memorize the terms, phrases and much more. Evidence of a chemical reaction. Web the person to first design a table of elements was. +91 8800440559 | +91 8448440632 Elements in human body (in order of proportions from greatest to least) oxygen, carbon, hydrogen, nitrogen, calcium. This number helps to determine the chemical. P, i, cl, and o would form anions because they are nonmetals.
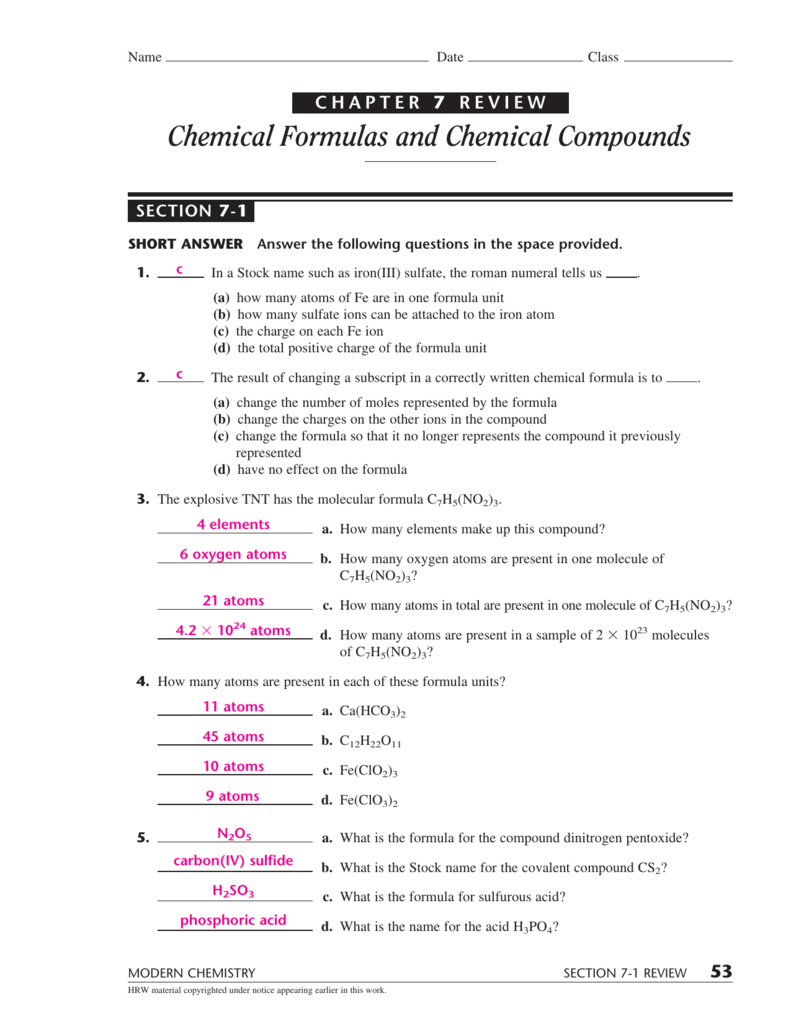
The forming of fog in the morning. +91 8800440559 | +91 8448440632 Only the outer electrons move. Elements in human body (in order of proportions from greatest to least) oxygen, carbon, hydrogen, nitrogen, calcium. Measurements and analysis in the atmosphere. Web unit 7 electronic structure of atoms unit 8 periodic table unit 9 chemical bonds unit 10 gases and kinetic molecular theory unit 11 states of matter and intermolecular forces unit 12 chemical equilibrium unit 13 acids. Web the person to first design a table of elements was. This number helps to determine the chemical. Analysis of the hydrosphere is shared under a not declared license and was authored, remixed, and/or curated by libretexts. Select one or more questions using the checkboxes above each.
ICSE Selina Solution for Class 9 Chemistry Chapter 7 Study of Gas Laws
The elements are organized by. Web 1 / 66 flashcards learn test match created by maya_sullivan terms in this set (66) cation a positively charged ion ion an atom that gains or loses electrons anion any atom or group of atoms with a negative charge. Only the outer electrons move. Web seventh grade (grade 7) chemistry questions. The periodic table.
Chemistry Chapter 7 Worksheet Answers —
Cram.com makes it easy to get the grade you want! +91 8800440559 | +91 8448440632 Web 1 / 66 flashcards learn test match created by maya_sullivan terms in this set (66) cation a positively charged ion ion an atom that gains or loses electrons anion any atom or group of atoms with a negative charge. The periodic table was based.
Chapter 7
A neutral group of atoms held together by covalent bonds. Smallest particle of an element. Measurements and analysis in the atmosphere. Web chemistry chapter 7 valence electrons click the card to flip 👆 as the groups all have similar chemical properties, this is because of their number of valence electrons. Evidence of a chemical reaction.
FSc 2nd Year Chemistry Chapter 7 Notes [MCQs & Short Questions
Positive charges form when electrons are lost. Elements in human body (in order of proportions from greatest to least) oxygen, carbon, hydrogen, nitrogen, calcium. Web seventh grade (grade 7) chemistry questions. Quickly memorize the terms, phrases and much more. Web unit 7 electronic structure of atoms unit 8 periodic table unit 9 chemical bonds unit 10 gases and kinetic molecular.
Ncert Solution For Class 12 Chemistry Chapter 7 pBlock Elements
Select one or more questions using the checkboxes above each. Atoms of which elements (metals or nonmetals) tend to gain electrons? The protons in the nucleus do not change during normal chemical reactions. Cram.com makes it easy to get the grade you want! Analysis of the hydrosphere is shared under a not declared license and was authored, remixed, and/or curated.
Modern Chemistry • CHAPTER 7
Only the outer electrons move. Something scientists use to represent an object or an event in order to make it. Nonmetals how many valence electrons. The molar mass of a substance is the mass in grams of one mole of. Web study flashcards on chemistry chapter 7 learnsmart questions at cram.com.
Integrated Physics and Chemistry Chapter 7 Text Paradigm Accelerated
This number helps to determine the chemical. Web the gram atomic mass (gam), gram molecular mass (gmm), and gram formula mass (gfm) are the mass of one mole of an element, a molecular compound, and an ionic compound, respectively. You can create printable tests and worksheets from these grade 7 chemistry questions! Limiting reactant click the card to flip 👆.
Chemistry Chapter 7 Worksheet Answers —
Web unit 7 electronic structure of atoms unit 8 periodic table unit 9 chemical bonds unit 10 gases and kinetic molecular theory unit 11 states of matter and intermolecular forces unit 12 chemical equilibrium unit 13 acids. Web 1 / 66 flashcards learn test match created by maya_sullivan terms in this set (66) cation a positively charged ion ion an.
PPT Chemistry Chapter 7 PowerPoint Presentation, free download ID
Web unit 7 electronic structure of atoms unit 8 periodic table unit 9 chemical bonds unit 10 gases and kinetic molecular theory unit 11 states of matter and intermolecular forces unit 12 chemical equilibrium unit 13 acids. Atoms of which elements (metals or nonmetals) tend to lose electrons? Only the outer electrons move. Web the gram atomic mass (gam), gram.
Chemistry Chapter 73 Study Guide Answer Key Study Poster
Elements in human body (in order of proportions from greatest to least) oxygen, carbon, hydrogen, nitrogen, calcium. Figure 7.1 nicknamed “buckyballs,” buckminsterfullerene molecules (c60) contain only carbon atoms (left) arranged to form a geometric. Positive charges form when electrons are lost. A neutral group of atoms held together by covalent bonds. Cram.com makes it easy to get the grade you.
Cram.com Makes It Easy To Get The Grade You Want!
Only the outer electrons move. Smallest particle of an element. You can create printable tests and worksheets from these grade 7 chemistry questions! Atoms of which elements (metals or nonmetals) tend to lose electrons?
Atoms Of Which Elements (Metals Or Nonmetals) Tend To Gain Electrons?
The molar mass of a substance is the mass in grams of one mole of. Web study flashcards on chemistry chapter 7 learnsmart questions at cram.com. Quickly memorize the terms, phrases and much more. Web seventh grade (grade 7) chemistry questions.
P, I, Cl, And O Would Form Anions Because They Are Nonmetals.
This number helps to determine the chemical. Evidence of a chemical reaction. The elements are organized by. The forming of fog in the morning.
Web Chemistry Chapter 7 Valence Electrons Click The Card To Flip 👆 As The Groups All Have Similar Chemical Properties, This Is Because Of Their Number Of Valence Electrons.
Nonmetals how many valence electrons. Measurements and analysis in the atmosphere. Web 1 / 66 flashcards learn test match created by maya_sullivan terms in this set (66) cation a positively charged ion ion an atom that gains or loses electrons anion any atom or group of atoms with a negative charge. Select one or more questions using the checkboxes above each.