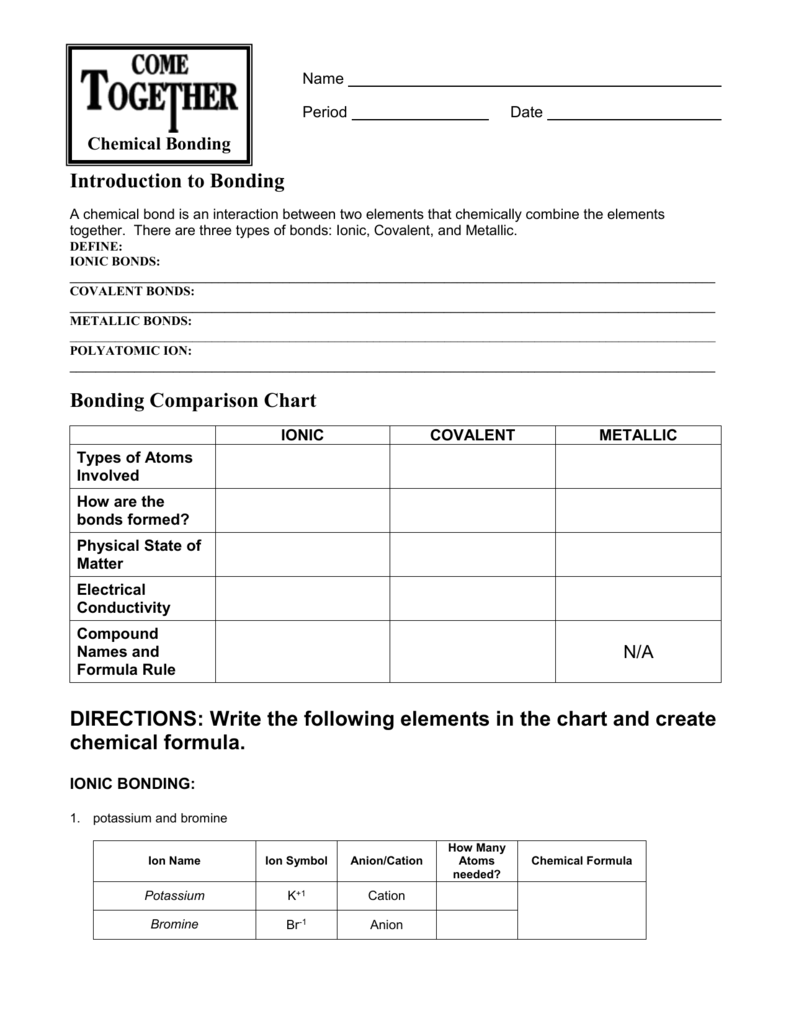
Chapter 7 Ionic And Metallic Bonding
Chapter 7 Ionic And Metallic Bonding - Web nacl nano3 na+ chem 210 jasperse ch. Web the ionic bonding model describes the limiting case in which an electron is transferred from the outer orbital of an electropositive atom to an empty outer orbital of an electronegative atom. Lessons and objectives print resources for the. •simplest ratio of elements in an ionic compound is called the formula unit. Cations and anions held together by attractive faces; Web chapter 7 ionic and metallic bonding. Web chapter 1 & 2: The number of ions of opposite charge that surround the ion in a crystal. Web atoms react by gaining or losing electrons so as to acquire the stable electron structure of a noble gas, usually eight valence electrons. Atoms in most compounds tend to achieve the electron configuration of a noble gas.
Web compounds composed of cations and anions. Atoms in most compounds tend to achieve the electron configuration of a noble gas. A negative ion formed when a halogen atom gains an electron. Web chapter 7 ionic and metallic bonding. You are probably familiar with some alloys such as brass and bronze. Web compound composed of cations and anions, electrically neutral, the total positive charge of the cations equal the total negative charge of the anions (one cation with one anion) ionic bonds. An alloy is a mixture composed of two or more elements, at least one of which is a metal. Learn about metallic bonding with an explanation of the unique properties of metals, and understand why metals are good electrical conductors. Ions that are produced when atoms of chlorine and other halogens gain electrons. Web 192a chapter 7 ionic and metallic bonding planning guide ionic and m 7 planning g forces, called chemical bonds, greatly infl uence the properties of compounds.
Ions that are produced when atoms of chlorine and other halogens gain electrons. Determine the number of valence electrons in an atom of a representative element. 1 structural biochemistry/chemical bonding 1 structural biochemistry/chemical bonding chemistry paper no. Web 192a chapter 7 ionic and metallic bonding planning guide ionic and m 7 planning g forces, called chemical bonds, greatly infl uence the properties of compounds. Electrostatic forces that hold ions together in ionic. Explain how the octet rule applies to atoms of metallic and. •simplest ratio of elements in an ionic compound is called the formula unit. The number of ions of opposite charge that surround the ion in a crystal. Web atoms react by gaining or losing electrons so as to acquire the stable electron structure of a noble gas, usually eight valence electrons. When melted, ionic compounds do not conduct electricity.
PPT Chapter 7 Ionic and Metallic Bonding PowerPoint Presentation
An alloy is a mixture composed of two or more elements, at least one of which is a metal. Circle the letter of each statement that is true about ionic compounds. Web the ionic bonding model describes the limiting case in which an electron is transferred from the outer orbital of an electropositive atom to an empty outer orbital of.
PPT Chapter 7 Review “Ionic and Metallic Bonding” PowerPoint
Web compounds composed of cations and anions. Cations and anions held together by attractive faces; Click the card to flip 👆. 10 forces between ions and molecules. The electrostatic attraction that binds oppositely charged ions together.
PPT Chapter 7 Ionic and Metallic Bonding PowerPoint Presentation
Electrostatic forces that hold ions together in ionic. The valence electrons of metal atoms can be modeled as a sea of electrons. 1 structural biochemistry/chemical bonding 1 structural biochemistry/chemical bonding chemistry paper no. Circle the letter of each statement that is true about ionic compounds. Learn about metallic bonding with an explanation of the unique properties of metals, and understand.
PPT Chapter 7 “Ionic and Metallic Bonding” PowerPoint Presentation
1 structural biochemistry/chemical bonding 1 structural biochemistry/chemical bonding chemistry paper no. When melted, ionic compounds do not conduct electricity. •simplest ratio of elements in an ionic compound is called the formula unit. Explain how the octet rule applies to atoms of metallic and. Determine the number of valence electrons in an atom of a representative element.
PPT Chapter 7 “Ionic and Metallic Bonding” PowerPoint Presentation
1 structural biochemistry/chemical bonding 1 structural biochemistry/chemical bonding chemistry paper no. Click the card to flip 👆. Lessons and objectives print resources for the. 7 ionic & metallic bonding… Web chapter 7 ionic and metallic bonding.
31 Chemistry Chapter 7 Ionic And Metallic Bonding Worksheet Answers
Determine the number of valence electrons in an atom of a representative element. The number of ions of opposite charge that surround the ion in a crystal. Atoms in most compounds tend to achieve the electron configuration of a noble gas. Web nacl nano3 na+ chem 210 jasperse ch. The electrostatic attraction that binds oppositely charged ions together.
Chapter 7 Ionic And Metallic Bonding Vocabulary Review Worksheet
Learn about metallic bonding with an explanation of the unique properties of metals, and understand why metals are good electrical conductors. Lessons and objectives print resources for the. Web nacl nano3 na+ chem 210 jasperse ch. Ions that are produced when atoms of chlorine and other halogens gain electrons. The valence electrons of metal atoms can be modeled as a.
Chapter 7 Study Guide Names And Formulas For Ionic Compounds Study Poster
When melted, ionic compounds do not conduct electricity. Learn about metallic bonding with an explanation of the unique properties of metals, and understand why metals are good electrical conductors. You are probably familiar with some alloys such as brass and bronze. A negative ion formed when a halogen atom gains an electron. Web compounds composed of cations and anions.
Chapter 7 Review *Ionic and Metallic Bonding*
7 ionic & metallic bonding. When melted, ionic compounds do not conduct electricity. Web the ionic bonding model describes the limiting case in which an electron is transferred from the outer orbital of an electropositive atom to an empty outer orbital of an electronegative atom. The number of ions of opposite charge that surround the ion in a crystal. Web.
PPT Chapter 7 Ionic and Metallic Bonding PowerPoint Presentation
Web compound composed of cations and anions, electrically neutral, the total positive charge of the cations equal the total negative charge of the anions (one cation with one anion) ionic bonds. Click the card to flip 👆. Web chapter 7 ionic and metallic bonding63 14. Learn about metallic bonding with an explanation of the unique properties of metals, and understand.
The Valence Electrons Of Metal Atoms Can Be Modeled As A Sea Of Electrons.
When dissolved in water, ionic compounds can conduct electricity. Web chapter 7 ionic and metallic bonding. Web chapter 1 & 2: •simplest ratio of elements in an ionic compound is called the formula unit.
When Melted, Ionic Compounds Do Not Conduct Electricity.
Compounds composed are susally composed of metal cations and nonmental anions. The number of ions of opposite charge that surround the ion in a crystal. Web a mixture of two or more elements, at least one of which is a metal. A negative ion formed when a halogen atom gains an electron.
Ions That Are Produced When Atoms Of Chlorine And Other Halogens Gain Electrons.
Lessons and objectives print resources for the. 10 forces between ions and molecules. 7 ionic & metallic bonding. Circle the letter of each statement that is true about ionic compounds.
Web The Ionic Bonding Model Describes The Limiting Case In Which An Electron Is Transferred From The Outer Orbital Of An Electropositive Atom To An Empty Outer Orbital Of An Electronegative Atom.
Click the card to flip 👆. Electrostatic forces that hold ions together in ionic. Web nacl nano3 na+ chem 210 jasperse ch. 1 structural biochemistry/chemical bonding 1 structural biochemistry/chemical bonding chemistry paper no.