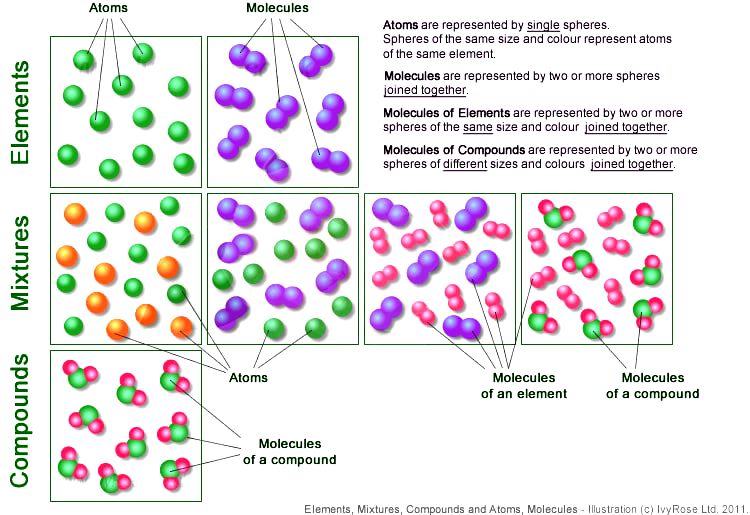
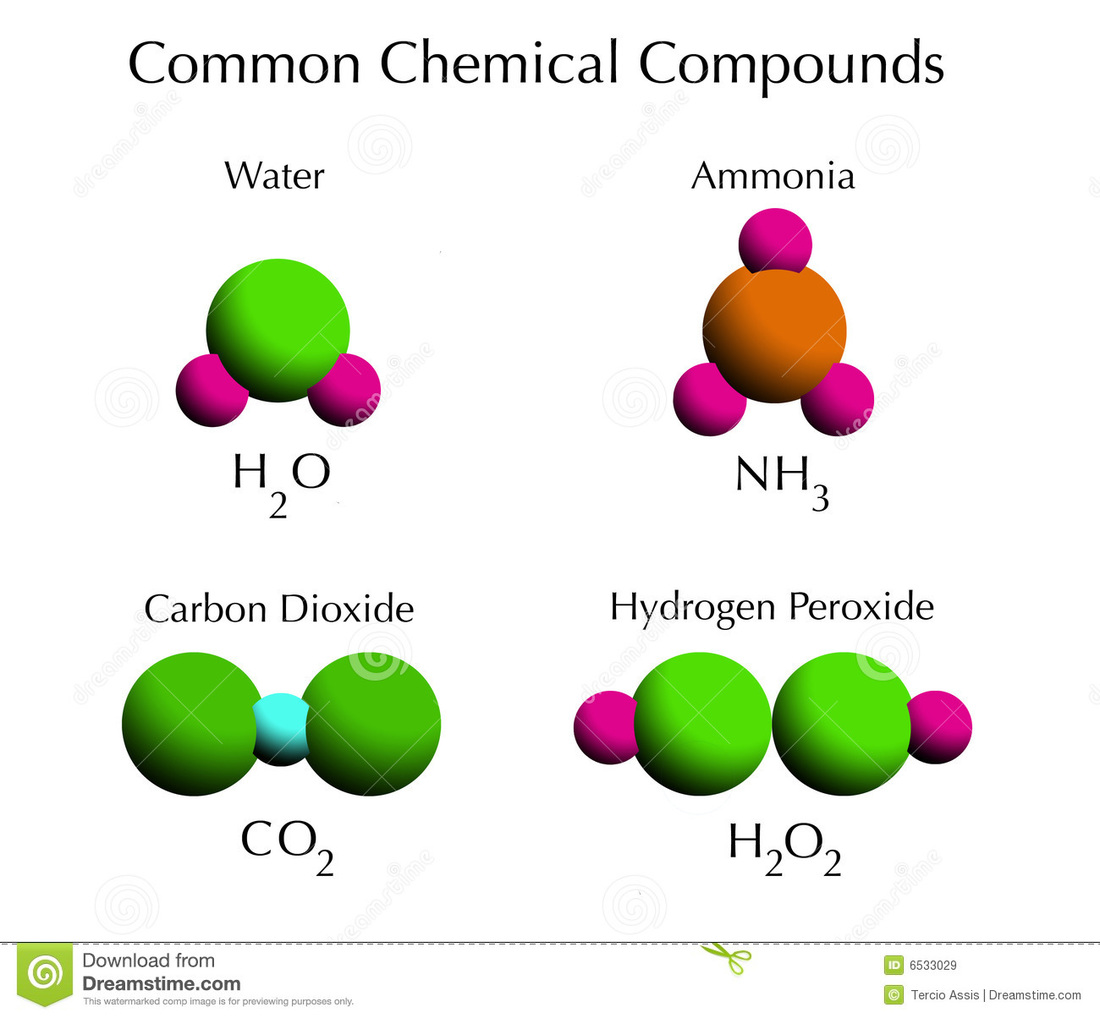
Chapter 6 Section 1 Atoms Elements And Compounds
Chapter 6 Section 1 Atoms Elements And Compounds - Web anonymous libretexts there were 2 categories of matter introduced in chapter 2 that we will look at in more detail here:. A pure substance formed when two or more. Compound a ________ is a. _____ are the building blocks of matter _____ are positively charged. Atoms, elements, and compounds in this section: Web chapter 6 study guide: Is a compound in which that atoms are held together by covalent bonds. Web atoms of the same element that have the same number of protons and electrons but have different number of neutrons. Web this chapter introduced some of the fundamental concepts of chemistry, with particular attention to the basic properties. Web the____ of an element is the amount of time it takes for the half of a radioactive isotope to decay.
Click the card to flip 👆. Web the____ of an element is the amount of time it takes for the half of a radioactive isotope to decay. Web chapter 6 section 1:atoms, elements, and compounds in your textbook, read about the structure of atoms. Atoms, elements, and compounds in this section: _____ are the building blocks of matter _____ are positively charged. Is a compound in which that atoms are held together by covalent bonds. The study of matter, its composition, and its properties. (a) chemists have put hydrogen. A pure substance formed when two or more. Web a mixture consists of two or more elements or compounds not chemically combined together.
Web a mixture consists of two or more elements or compounds not chemically combined together. _____ are the building blocks of matter _____ are positively charged. Web biology chapter 6: Atoms, elements, and compounds in this section: Atoms, elements, and compounds in this section: Web study with quizlet and memorize flashcards containing terms like remember that a ___ is made of the atoms of two or more. Web anonymous libretexts there were 2 categories of matter introduced in chapter 2 that we will look at in more detail here:. Click the card to flip 👆. Web chapter 6 section 1:atoms, elements, and compounds in your textbook, read about the structure of atoms. Is a compound in which that atoms are held together by covalent bonds.
Atoms, elements and compounds academy
Web anonymous libretexts there were 2 categories of matter introduced in chapter 2 that we will look at in more detail here:. Click the card to flip 👆. Web a mixture consists of two or more elements or compounds not chemically combined together. Is a compound in which that atoms are held together by covalent bonds. Web by the end.
CH3 ATOM, ELEMENTS & COMPOUNDS
Web chapter 6 section 1:atoms, elements, and compounds in your textbook, read about the structure of atoms. Web study with quizlet and memorize flashcards containing terms like define ionic bond, identify the particles that make up atoms,. A pure substance formed when two or more. Compound a ________ is a. Web atoms of the same element that have the same.
Chapter 6 Section 1 Atoms Elements and Compounds YouTube
Web the____ of an element is the amount of time it takes for the half of a radioactive isotope to decay. Web by the end of this section, you will be able to: The study of matter, its composition, and its properties. Web study with quizlet and memorize flashcards containing terms like remember that a ___ is made of the.
Atoms, elements, compounds and mixtures Crossword WordMint
Compound a ________ is a. Web by the end of this section, you will be able to: _____ are the building blocks of matter _____ are positively charged. Describe the basic properties of each physical state of matter: A pure substance formed when two or more.
1B3 Atoms and Molecules
Web as you have seen in this chapter, hydrogen is very different from the other elements in group 1. Click the card to flip 👆. Atoms, elements, and compounds in this section: Web anonymous libretexts there were 2 categories of matter introduced in chapter 2 that we will look at in more detail here:. _____ are the building blocks of.
Atoms, Elements, and Compound Test Study Guide
Web atoms of the same element that have different numbers of neutrons. Web atoms of the same element that have the same number of protons and electrons but have different number of neutrons. Web a mixture consists of two or more elements or compounds not chemically combined together. Is a compound in which that atoms are held together by covalent.
Chemistry Of Life Worksheet 1 Answer Key
Atoms, elements, and compounds in this section: Web this chapter introduced some of the fundamental concepts of chemistry, with particular attention to the basic properties. Web biology chapter 6: Web the____ of an element is the amount of time it takes for the half of a radioactive isotope to decay. Web the periodic table is organized into horizontal rows called.
Atoms, Elements, & Compounds
Atoms, elements, and compounds in this section: _____ are the building blocks of matter _____ are positively charged. Web the____ of an element is the amount of time it takes for the half of a radioactive isotope to decay. Web by the end of this section, you will be able to: The study of matter, its composition, and its properties.
Chemistry In Biology Chapter 6 Section 4 Study Guide Answers Study Poster
Web study with quizlet and memorize flashcards containing terms like remember that a ___ is made of the atoms of two or more. Compound a ________ is a. Atoms, elements, and compounds in this section: Web the____ of an element is the amount of time it takes for the half of a radioactive isotope to decay. Web biology chapter 6:
5, Atomic Structure and the Periodic Table
Atoms, elements, and compounds in this section: Web as you have seen in this chapter, hydrogen is very different from the other elements in group 1. Web this chapter introduced some of the fundamental concepts of chemistry, with particular attention to the basic properties. Is a compound in which that atoms are held together by covalent bonds. Describe the basic.
Web The____ Of An Element Is The Amount Of Time It Takes For The Half Of A Radioactive Isotope To Decay.
_____ are the building blocks of matter _____ are positively charged. Web as you have seen in this chapter, hydrogen is very different from the other elements in group 1. A pure substance formed when two or more. Web atoms of the same element that have the same number of protons and electrons but have different number of neutrons.
Click The Card To Flip 👆.
Web a mixture consists of two or more elements or compounds not chemically combined together. The study of matter, its composition, and its properties. Atoms, elements, and compounds in this section: Compound a ________ is a.
(A) Chemists Have Put Hydrogen.
Anything that has mass and takes up. Web chapter 6 section 1:atoms, elements, and compounds in your textbook, read about the structure of atoms. Describe the basic properties of each physical state of matter: Web atoms of the same element that have different numbers of neutrons.
Is A Compound In Which That Atoms Are Held Together By Covalent Bonds.
Web chapter 6 study guide: Web study with quizlet and memorize flashcards containing terms like define ionic bond, identify the particles that make up atoms,. Web by the end of this section, you will be able to: Web this chapter introduced some of the fundamental concepts of chemistry, with particular attention to the basic properties.