Chapter 2 Lesson 1 The Nature Of Matter Answer Key
Chapter 2 Lesson 1 The Nature Of Matter Answer Key - Explain how compounds are different from their component elements. Web 2.1 the nature of matter lesson objectives. Complete the table about subatomic particles. Located outside the atomic nucleus. There are an equal number of protons and electrons, so the charges balance out. Web one useful way of organizing our understanding of matter is to think of a hierarchy that extends down from the most general and complex to the simplest and most fundamental. The basic unit of matter. Just as buildings are made from bricks, steel,. Web the nature of matter. Web chapter 2 ##### lesson.
Web 2.1 the nature of matter lesson objectives identify the three subatomic particles found in atoms. Web carbon has an atomic number of _____. Web this lesson follows along with the miller & levine biology textbook chapter 2, lesson 1: Is the negatively charged particle; When you eat food or inhale oxygen, your body uses these materials in c hemical reactions that keep you alive. Is the basic unit of matter. Quickly memorize the terms, phrases and much more. There are an equal number of protons and electrons, so the charges balance out. Web study with quizlet and memorize flashcards containing terms like what is the study of compounds without carbon?, what is anything with mass and takes up space?, what is a pure substance with only 1 type. Web 2.1 the nature of matter class date atoms 1.
The basic unit of matter is called a(an) atom. Build vocabulary the chart below shows key terms from the lesson. When you eat food or inhale oxygen, your body uses these materials in c hemical reactions that keep you alive. Chapter 2.1 assessment the nature of matter. The nature of matter (worksheet/notes) 10 terms. Web neutrons are not positively charged or negatively charged, instead they are neutral particles within the nucleus of an atom. Web answer the following question to test your understanding of the preceding section:\ briefly contrast hypothalamic control of the anterior pituitary with its control of the posterior pituitary. Subtract the amount of protons from that number and what’s left are the neutrons. Web only $35.99/year the nature of matter (chapter 2,lesson 1) flashcards learn test match flashcards learn test match created by terms in this set (10) atom the basic unit of matter nucleus the center of an atom,. Web the nature of matter.
Chapter 2.1, 2.2 Review Packet Answer Key
Build vocabulary the chart below shows key terms from the lesson. Terms in this set (15) atom. Subtract the amount of protons from that number and what’s left are the neutrons. Complete the table about subatomic particles. The number of neutrons in the nucleus of an element can be identified by the atomic mass.
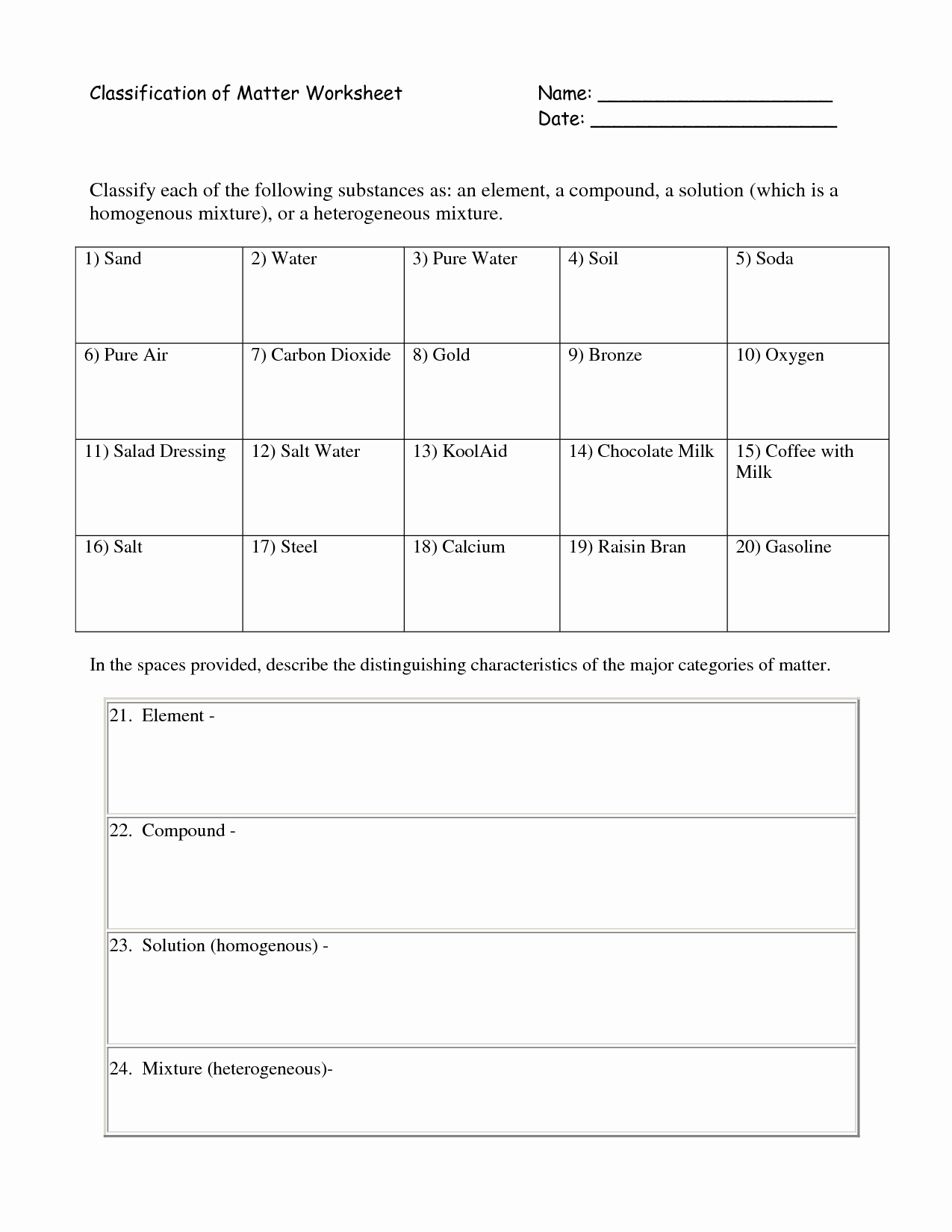
50 Classifying Matter Worksheet Answer Key Chessmuseum Template Library
This means every carbon _____ in the _____ has 6 protons in its _____. Web study with quizlet and memorize flashcards containing terms like what is the study of compounds without carbon?, what is anything with mass and takes up space?, what is a pure substance with only 1 type. Denote the atomic number of the atom; The number of.
30 Classifying Matter Worksheet Answer Key Education Template
Web neutrons are not positively charged or negatively charged, instead they are neutral particles within the nucleus of an atom. Web 2.1 the nature of matter class date atoms 1. Cram.com makes it easy to. It is the center of an atom, made up of protons and neutrons. Terms in this set (15) atom.
Chapter 1 The Nature of Science STUDY GUIDE Answer Key
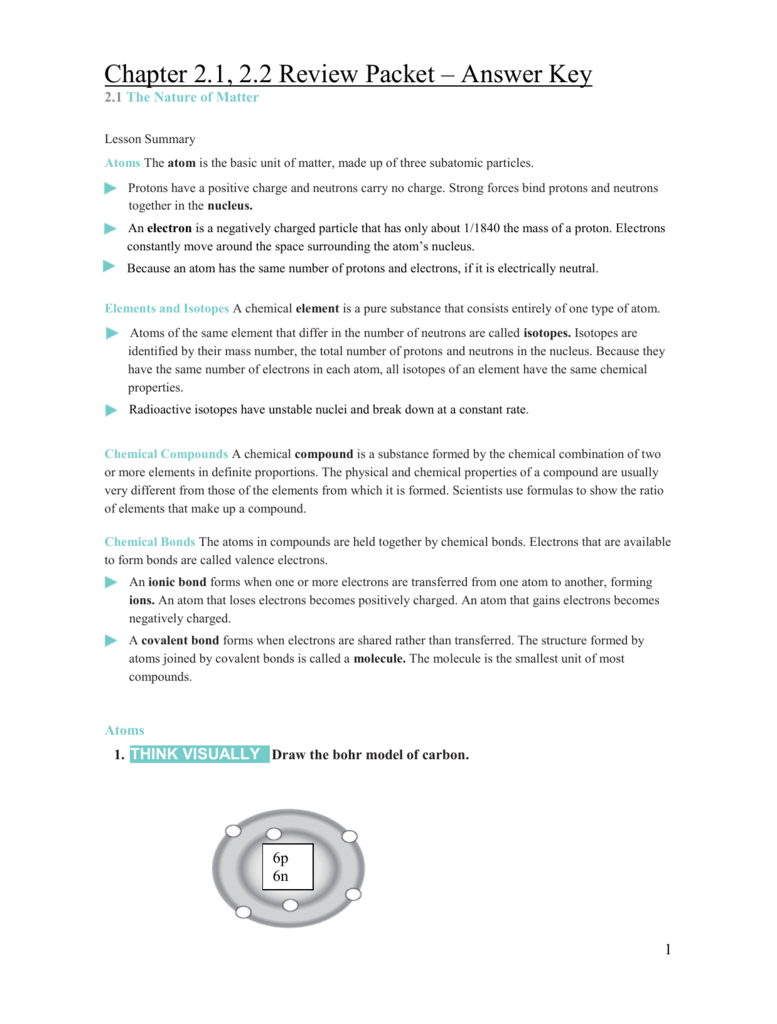
1 the nature of matter. Just as buildings are made from bricks, steel,. Denote the atomic number of the atom; Label all particles and the nucleus. Complete the diagram by drawing in the rest of the atomic particles, including their charges.
30 Classifying Matter Worksheet Answer Key Education Template
Label all particles and the nucleus. Located outside the atomic nucleus. Complete the table about subatomic particles. Chapter 2.1 assessment the nature of matter. Web chapter 2 ##### lesson.
2.1 The Nature of Matter
Web the nature of matter learn with flashcards, games, and more — for free. Web this lesson follows along with the miller & levine biology textbook chapter 2, lesson 1: Web carbon has an atomic number of _____. Quickly memorize the terms, phrases and much more. Terms in this set (15) atom.
Hei! 29+ Sannheter du Ikke Visste om States Of Matter Phet Answer Key
Is the negatively charged particle; Terms in this set (15) atom. Label all particles and the nucleus. Web carbon has an atomic number of _____. How do gecko’s climb vertical surface?
42 chapter 2 matter and change worksheet answers pearson Worksheet Online
Label all particles and the nucleus. Web this lesson follows along with the miller & levine biology textbook chapter 2, lesson 1: Subtract the amount of protons from that number and what’s left are the neutrons. Web study with quizlet and memorize flashcards containing terms like what is the study of compounds without carbon?, what is anything with mass and.
30 Classifying Matter Worksheet Answer Key Education Template
1 the nature of matter. Cram.com makes it easy to. Build vocabulary the chart below shows key terms from the lesson. Complete the diagram by drawing in the rest of the atomic particles, including their charges. Web 2.1 the nature of matter lesson objectives.
Chemistry Worksheet Matter 1 Answers
Subtract the amount of protons from that number and what’s left are the neutrons. Just as buildings are made from bricks, steel,. Material covered in this lesson:→types of matter→atoms→bondingin this resource, you will get:→google slides slideshow→powerpoint→guided notes that follow the slideshow→a key for the not. The basic unit of matter. Terms in this set (15) atom.
Web Why Are Atoms Electronically Neutral?
It is the center of an atom, made up of protons and neutrons. Web one useful way of organizing our understanding of matter is to think of a hierarchy that extends down from the most general and complex to the simplest and most fundamental. Describe the nucleus of an atom. There are an equal number of protons and electrons, so the charges balance out.
Explain How Compounds Are Different From Their Component Elements.
When you eat food or inhale oxygen, your body uses these materials in c hemical reactions that keep you alive. Chemical element a pure substance that consists entirely of one type of atom how. The basic unit of matter. The nature of matter (worksheet/notes) 10 terms.
This Means Every Carbon _____ In The _____ Has 6 Protons In Its _____.
Web the nature of matter. Just as buildings are made from bricks, steel,. Web neutrons are not positively charged or negatively charged, instead they are neutral particles within the nucleus of an atom. Terms in this set (15) atom.
Web Chapter 2 ##### Lesson.
Web kw_123445 terms in this set (21) proton, neutron, and electron three subatomic particles that make up an atom protons have a positive electrical charge; Web answer the following question to test your understanding of the preceding section:\ briefly contrast hypothalamic control of the anterior pituitary with its control of the posterior pituitary. Is the basic unit of matter. Is the center of the atom which contains the protons and neutrons.