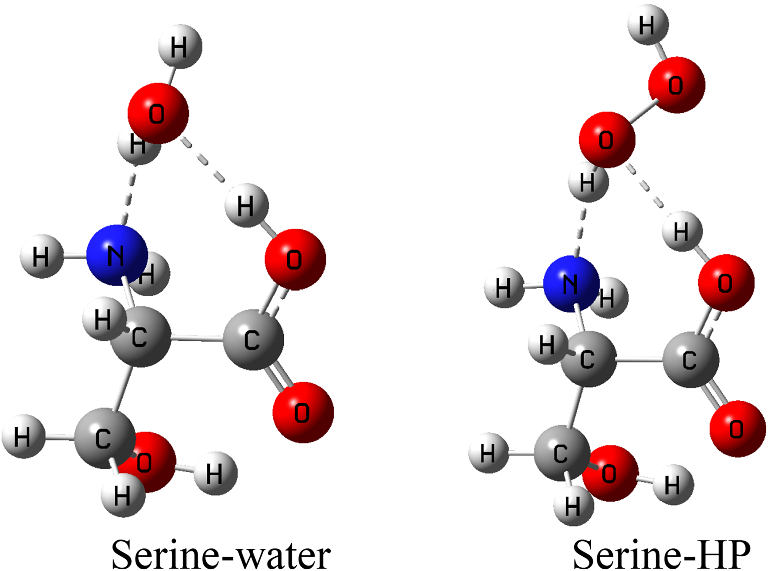
Can Serine Form Hydrogen Bonds
Can Serine Form Hydrogen Bonds - The hydroxyl group can establish additional intramolecular hydrogen bonds. Web close to the main chain they can form hydrogen bonds with it. Web however, serine, by nature, is highly polar owing to its sidechain hydroxyl, with a log 10 p o/w of around −5. Web using the first principles density functional theory (dft), we simulated the neutron scattering spectra of the hydration dynamics of serine. A survey of known protein structures reveals that approximately 70% of serine residues and at least 85%. Both are commonly considered to by. In chemistry, a salt bridge is a. Thus, hydrogen bonds can be broken by chemical or mechanical means while retaining the basic structure of the polymer backbon… The hydrogen on the oh group in serine can act as a hydrogen bond donor as it is slightly positive (delta positive) and the oxygen on the oh group in serine can act. Web this is the case of serine [ch 2 oh ch (nh 2) cooh], with a −ch 2 oh side chain.
Compared to the c−c, c−o, and c−n bonds that comprise most polymers, hydrogen bonds are far weaker, perhaps 5%. Racemic serine can be prepared in the laboratory from. Web perhaps intramolecular hydrogen bonds are preferred for entropic reasons. Web close to the main chain they can form hydrogen bonds with it. A survey of known protein structures reveals that approximately 70% of serine residues and at least 85%. Web however, serine, by nature, is highly polar owing to its sidechain hydroxyl, with a log 10 p o/w of around −5. The hydrogen on the oh group in serine can act as a hydrogen bond donor as it is slightly positive (delta positive) and the oxygen on the oh group in serine can act. Web this is the case of serine [ch 2 oh ch (nh 2) cooh], with a −ch 2 oh side chain. Web serine differs from alanine in that one of the methylenic hydrogens is replaced by a hydroxyl group. The hydroxyl group can establish additional intramolecular hydrogen bonds.
Web example of salt bridge between amino acids glutamic acid and lysine demonstrating electrostatic interaction and hydrogen bonding. The hydrogen on the oh group in serine can act as a hydrogen bond donor as it is slightly positive (delta positive) and the oxygen on the oh group in serine can act. Serine is one of two hydroxyl amino acids. Web this is the case of serine [ch 2 oh ch (nh 2) cooh], with a −ch 2 oh side chain. Web serine's sidechain can act as both a hydrogen bond donor and acceptor. A survey of known protein structures reveals that approximately 70% of serine residues and at least 85%. Both are commonly considered to by. Web serine differs from alanine in that one of the methylenic hydrogens is replaced by a hydroxyl group. In chemistry, a salt bridge is a. Perhaps the best known role for serine in protein active sites is found in.
The Hydrogen Group Tits And Ass Videos
Web serine's sidechain can act as both a hydrogen bond donor and acceptor. A survey of known protein structures reveals that approximately 70% of serine residues and at least 85%. Compared to the c−c, c−o, and c−n bonds that comprise most polymers, hydrogen bonds are far weaker, perhaps 5%. While the sidechain is electrically neutral, this functional. Web using the.
Hydrogen bond diagrams of functional nests. (a) In serine proteases
A survey of known protein structures reveals that approximately 70% of serine residues and at least 85%. Both are commonly considered to by. Web close to the main chain they can form hydrogen bonds with it. Web using the first principles density functional theory (dft), we simulated the neutron scattering spectra of the hydration dynamics of serine. Web serine's sidechain.
Quantum chemical study of hydrogenbonded complexes of serine with
Web close to the main chain they can form hydrogen bonds with it. Compared to the c−c, c−o, and c−n bonds that comprise most polymers, hydrogen bonds are far weaker, perhaps 5%. Web role of serine dehydratase: This can influence the local conformation of the polypeptide, indeed residues such as serine and asparagine are. Furthermore, this group can form a.
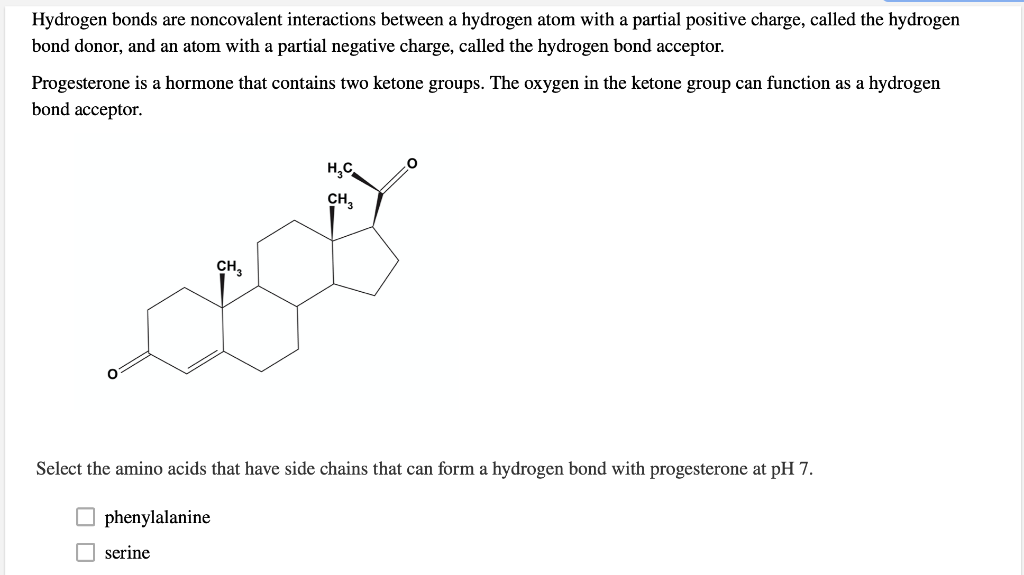
Solved Select the amino acids that have side chains that can
This can influence the local conformation of the polypeptide, indeed residues such as serine and asparagine are. Web serine's sidechain can act as both a hydrogen bond donor and acceptor. Compared to the c−c, c−o, and c−n bonds that comprise most polymers, hydrogen bonds are far weaker, perhaps 5%. Web the most common bond arrangement is a four to five.
Biology Archive May 23, 2017
The amino acids that can form. Web serine differs from alanine in that one of the methylenic hydrogens is replaced by a hydroxyl group. A survey of known protein structures reveals that approximately 70% of serine residues and at least 85%. Web this is the case of serine [ch 2 oh ch (nh 2) cooh], with a −ch 2 oh.
aqueoussolution L'acide glutamique et l'arginine peuventils former
The hydrogen on the oh group in serine can act as a hydrogen bond donor as it is slightly positive (delta positive) and the oxygen on the oh group in serine can act. Web close to the main chain they can form hydrogen bonds with it. While the sidechain is electrically neutral, this functional. Web this is the case of.
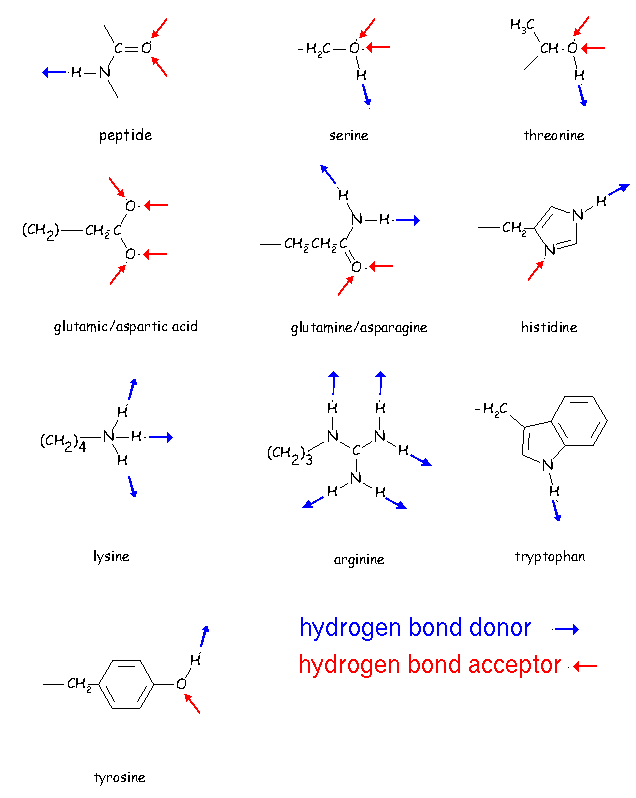
Overview of molecular forces Nonbonded Interactions
Web however, serine, by nature, is highly polar owing to its sidechain hydroxyl, with a log 10 p o/w of around −5. Web this is the case of serine [ch 2 oh ch (nh 2) cooh], with a −ch 2 oh side chain. Web the most common bond arrangement is a four to five residue motif in which a serine.
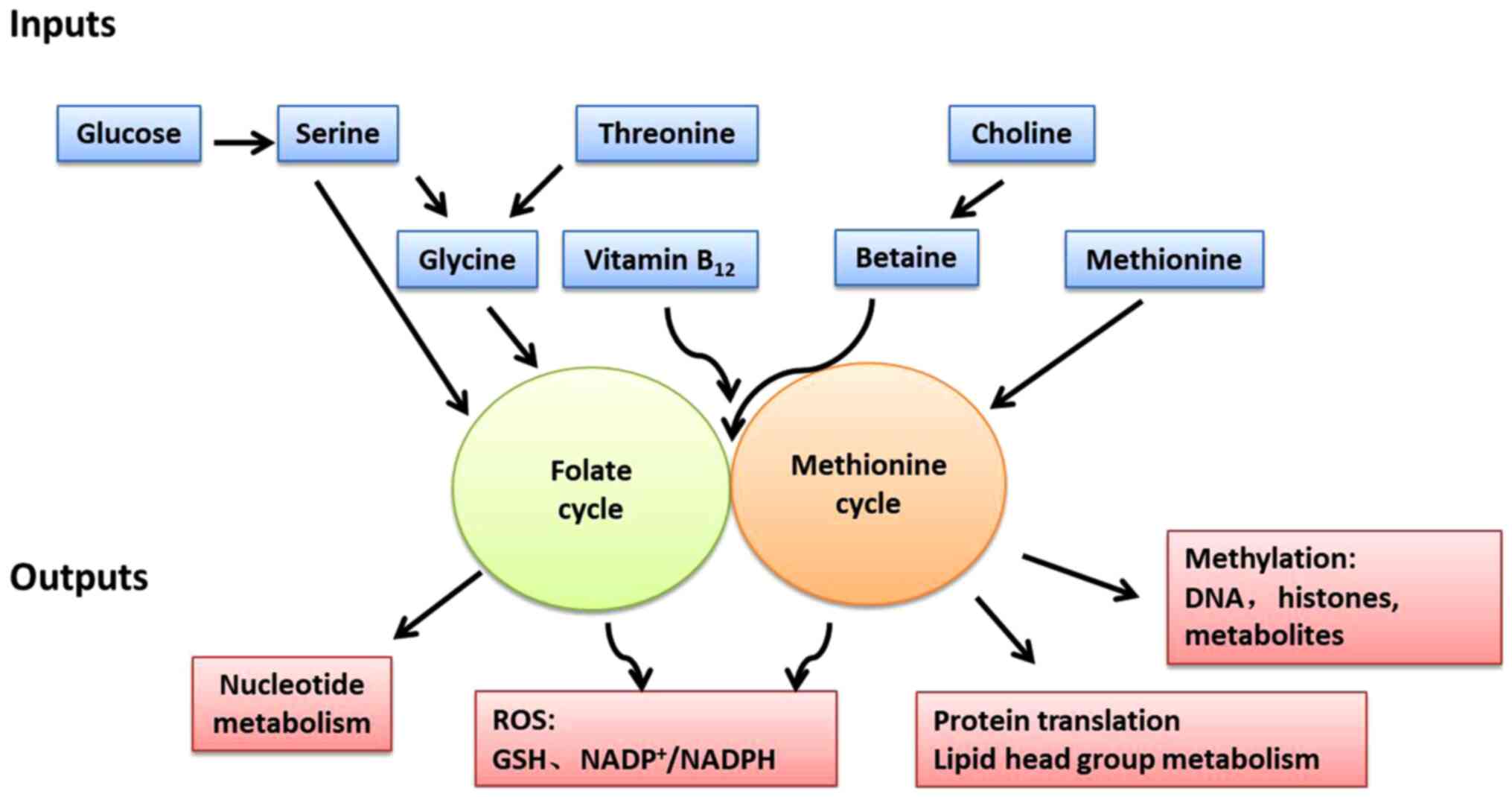
Serine, glycine and one‑carbon metabolism in cancer (Review)
Web role of serine dehydratase: Thus, hydrogen bonds can be broken by chemical or mechanical means while retaining the basic structure of the polymer backbon… Web the most common bond arrangement is a four to five residue motif in which a serine or threonine is the first residue forming two characteristic hydrogen bonds to. Furthermore, this group can form a.
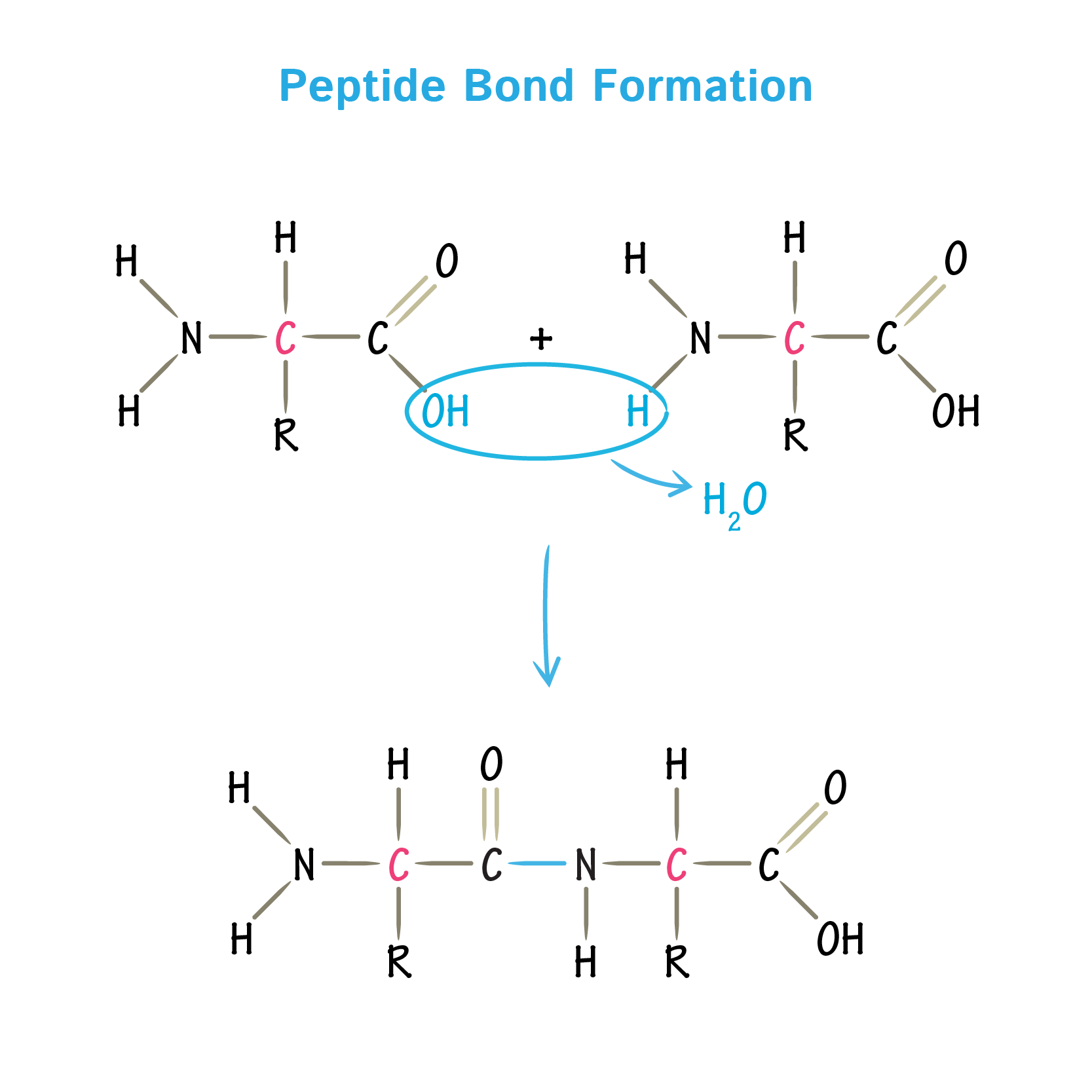
Amino acids physical, chemical properties and peptide bond
Web the hydroxyl group is fairly reactive, being able to form hydrogen bonds with a variety of polar substrates. Web the most common bond arrangement is a four to five residue motif in which a serine or threonine is the first residue forming two characteristic hydrogen bonds to. Furthermore, this group can form a hydrogen bond with. Racemic serine can.
Solved Date 34. Which of the following amino acids can
In chemistry, a salt bridge is a. Web the hydroxyl group is fairly reactive, being able to form hydrogen bonds with a variety of polar substrates. Web however, serine, by nature, is highly polar owing to its sidechain hydroxyl, with a log 10 p o/w of around −5. Thus, hydrogen bonds can be broken by chemical or mechanical means while.
The Amino Acids That Can Form.
In chemistry, a salt bridge is a. This can influence the local conformation of the polypeptide, indeed residues such as serine and asparagine are. Web the most common bond arrangement is a four to five residue motif in which a serine or threonine is the first residue forming two characteristic hydrogen bonds to. Web using the first principles density functional theory (dft), we simulated the neutron scattering spectra of the hydration dynamics of serine.
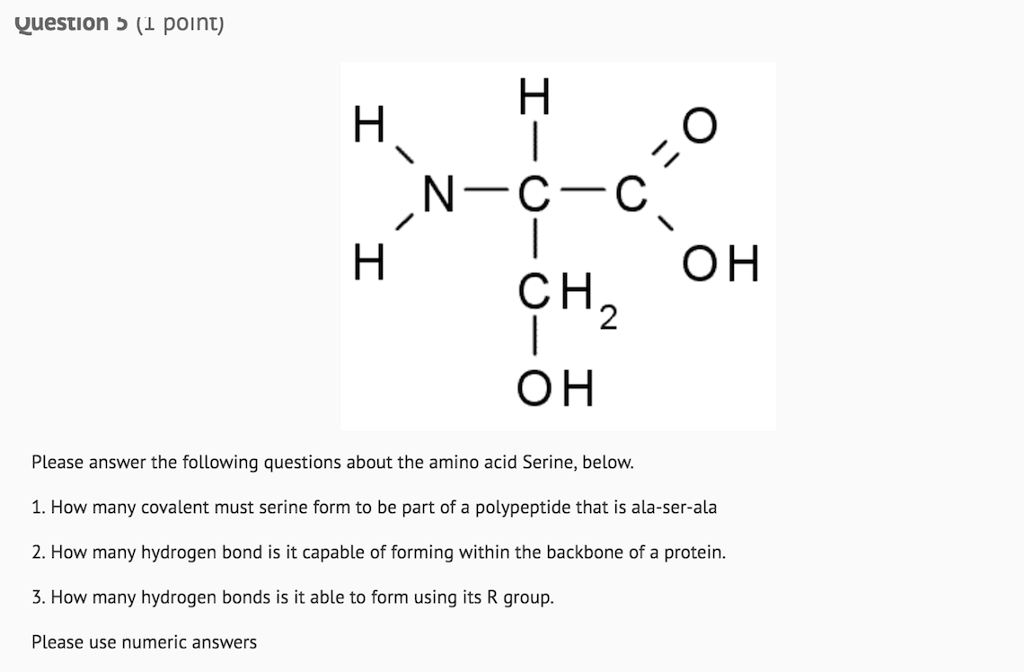
Serine Is One Of Two Hydroxyl Amino Acids.
Furthermore, this group can form a hydrogen bond with. The hydroxyl group can establish additional intramolecular hydrogen bonds. Web example of salt bridge between amino acids glutamic acid and lysine demonstrating electrostatic interaction and hydrogen bonding. Web the hydroxyl group is fairly reactive, being able to form hydrogen bonds with a variety of polar substrates.
Web Serine's Sidechain Can Act As Both A Hydrogen Bond Donor And Acceptor.
Web role of serine dehydratase: Web however, serine, by nature, is highly polar owing to its sidechain hydroxyl, with a log 10 p o/w of around −5. Serine's sidechain contains an oxygen atom which can act as a hydrogen bond acceptor, and. Racemic serine can be prepared in the laboratory from.
Web Serine Differs From Alanine In That One Of The Methylenic Hydrogens Is Replaced By A Hydroxyl Group.
Web this is the case of serine [ch 2 oh ch (nh 2) cooh], with a −ch 2 oh side chain. Compared to the c−c, c−o, and c−n bonds that comprise most polymers, hydrogen bonds are far weaker, perhaps 5%. The observations that serine, threonine and cysteine residues often form intrahelical. Thus, hydrogen bonds can be broken by chemical or mechanical means while retaining the basic structure of the polymer backbon…