Can Hf Form Hydrogen Bonds
Can Hf Form Hydrogen Bonds - Web on average, then, each molecule can only form one hydrogen bond using its δ+ hydrogen and one involving one of its lone pairs. Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. The other lone pairs are essentially. Web hydrogen bonding between hf molecules is particularly evident in solid hf, where the atoms are arranged in a zigzag pattern: Web as a liquid, hf forms relatively strong hydrogen bonds, hence its relatively high boiling point. Each water molecule forms 4 hydrogen bonding. Molecules with hydrogen bonds will. Web hydrogen bonds form between the δ+ hydrogen on one hf molecule and a lone pair on the fluorine of another one.the figure below illustrates this association: According to my information, hydrogen bonding present in ice. But in gas phase , molecules are far apart.
Web answer (1 of 3): Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. Such a bond is weaker than an ionic bond or. The total number of hydrogen. The other lone pairs are essentially. Molecules with hydrogen bonds will. But in gas phase , molecules are far apart. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; According to my information, hydrogen bonding present in ice. The hf molecules, with a.
Web on average, then, each molecule can only form one hydrogen bond using its δ+ hydrogen and one involving one of its lone pairs. The hf molecules, with a. Web hydrofluoric acid (hf): Such a bond is weaker than an ionic bond or. Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. Web hydrogen bonding between hf molecules is particularly evident in solid hf, where the atoms are arranged in a zigzag pattern: The other lone pairs are essentially. Web hydrogen bonds also occur when hydrogen is bonded to fluorine, but the hf group does not appear in other molecules. The total number of hydrogen. Web in hf each molecule has one hydrogen atom which can form a hydrogen bond, and there are three lone pairs of electrons on the fluorine atom.
How do Hydrogen bonds form in H2O NH3 HF Hydrogen Bonding
Such a bond is weaker than an ionic bond or. The other lone pairs are essentially. Web that project will use 4 gigawatts of solar, wind power and storage to power a 2.2 gigawatt electrolyser that will produce up to 600 tonnes a day of hydrogen by the. Molecules with hydrogen bonds will. Web as a liquid, hf forms relatively.
Bonds That Hold Water Molecules Together / Intermolecular Forces
Web answer (1 of 3): Web hydrogen bonding between hf molecules is particularly evident in solid hf, where the atoms are arranged in a zigzag pattern: Such a bond is weaker than an ionic bond or. Web hydrofluoric acid (hf): Molecules with hydrogen bonds will.
Diagram Of Water Molecules Hydrogen Bonding
Each water molecule forms 4 hydrogen bonding. [17] [18] typical enthalpies in vapor include: Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Web in hf each molecule has one hydrogen atom which can form a hydrogen bond, and there are three lone pairs of electrons on the.
Hydrogen A Hydrogen Bond Is
The hf molecules, with a. Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. The other lone pairs are essentially. Web hydrogen bonds form between the δ+ hydrogen on one hf molecule and a lone pair on the fluorine of another one.the figure below illustrates this association: Web that project will.
chemistry Intermolecular Hydrogen Bonding
Web in hf each molecule has one hydrogen atom which can form a hydrogen bond, and there are three lone pairs of electrons on the fluorine atom. But in gas phase , molecules are far apart. Web hydrofluoric acid (hf): Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. Web hydrogen.
Why do H2O Molecules form more Hydrogen Bonds compared to NH3 and HF
Web on average, then, each molecule can only form one hydrogen bond using its δ+ hydrogen and one involving one of its lone pairs. Web hydrogen bonds also occur when hydrogen is bonded to fluorine, but the hf group does not appear in other molecules. Web hydrofluoric acid (hf): Hydrofluoric acid forms what is called a symmetric hydrogen bond, which.
Properties of Water Presentation Biology
Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; According to my information, hydrogen bonding present in ice. Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. Each water molecule forms 4 hydrogen bonding. Web in hf each molecule.
How can the hydrogen bonds in solid HF be best represented? ECHEMI
Molecules with hydrogen bonds will. Such a bond is weaker than an ionic bond or. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; But in gas phase , molecules are far apart. Web as a liquid, hf forms relatively strong hydrogen bonds, hence its relatively high boiling.
How many hydrogen bonds form by Nh3, H20 and HF and boiling point trend
Web hydrofluoric acid (hf): But in gas phase , molecules are far apart. Such a bond is weaker than an ionic bond or. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Web in hf each molecule has one hydrogen atom which can form a hydrogen bond, and.
chemical bonding hydrogen bonding notes for neet and jee
[17] [18] typical enthalpies in vapor include: The total number of hydrogen. Such a bond is weaker than an ionic bond or. Web hydrogen bonds form between the δ+ hydrogen on one hf molecule and a lone pair on the fluorine of another one.the figure below illustrates this association: The hf molecules, with a.
The Hf Molecules, With A.
Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Web hydrofluoric acid (hf): Web as a liquid, hf forms relatively strong hydrogen bonds, hence its relatively high boiling point. The other lone pairs are essentially.
But In Gas Phase , Molecules Are Far Apart.
Such a bond is weaker than an ionic bond or. The total number of hydrogen. Web hydrogen bonding between hf molecules is particularly evident in solid hf, where the atoms are arranged in a zigzag pattern: Each water molecule forms 4 hydrogen bonding.
Molecules With Hydrogen Bonds Will.
[17] [18] typical enthalpies in vapor include: According to my information, hydrogen bonding present in ice. Web hydrogen bonds also occur when hydrogen is bonded to fluorine, but the hf group does not appear in other molecules. Web answer (1 of 3):
Web On Average, Then, Each Molecule Can Only Form One Hydrogen Bond Using Its Δ+ Hydrogen And One Involving One Of Its Lone Pairs.
Web in hf each molecule has one hydrogen atom which can form a hydrogen bond, and there are three lone pairs of electrons on the fluorine atom. Web that project will use 4 gigawatts of solar, wind power and storage to power a 2.2 gigawatt electrolyser that will produce up to 600 tonnes a day of hydrogen by the. Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. Web hydrogen bonds form between the δ+ hydrogen on one hf molecule and a lone pair on the fluorine of another one.the figure below illustrates this association:



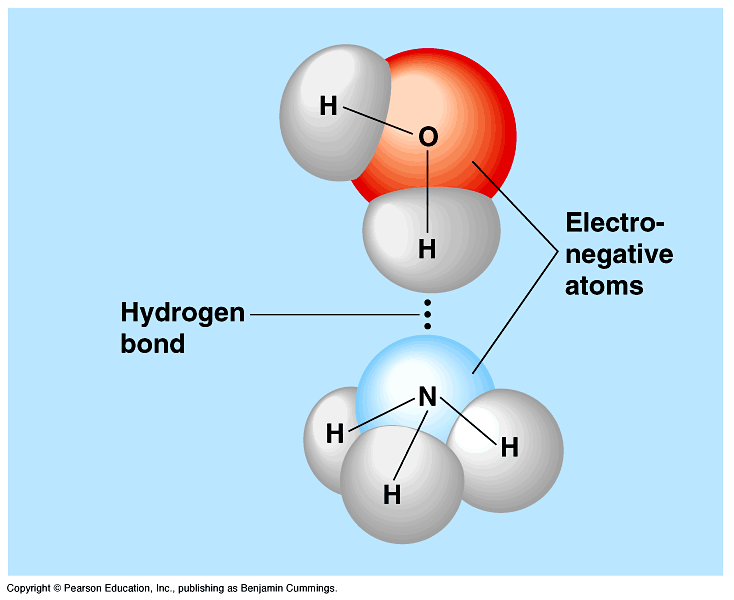


.PNG)


