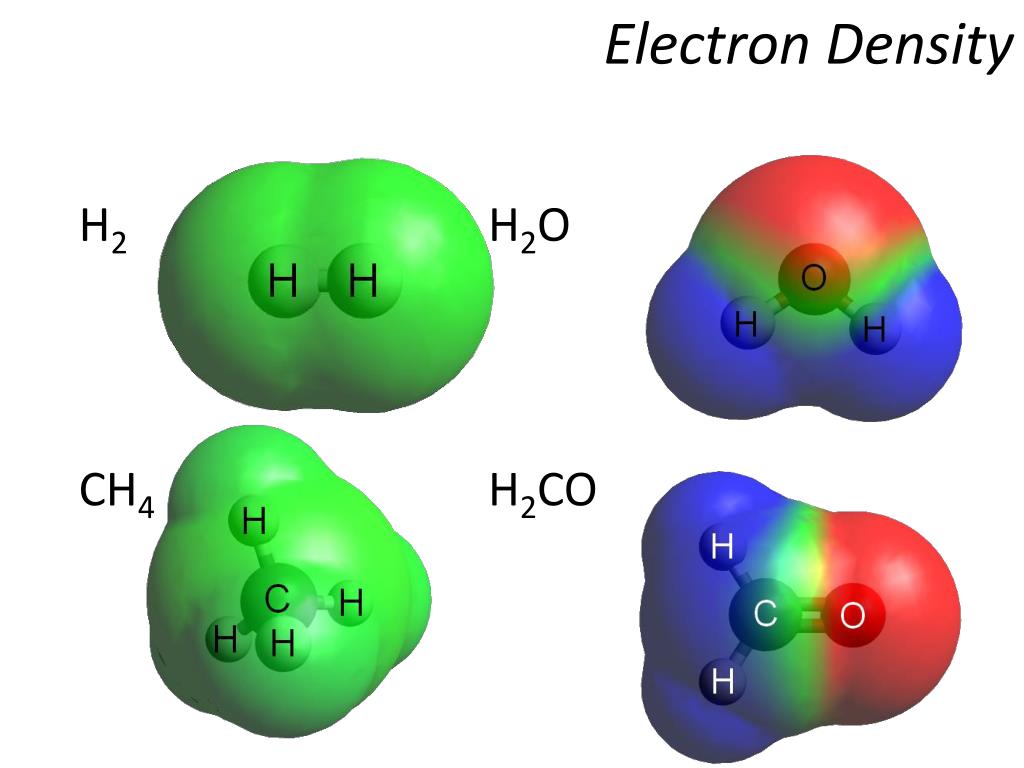
Can Ch4 Form Hydrogen Bonds
Can Ch4 Form Hydrogen Bonds - Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web can ch4 form hydrogen bonds? Web can ch4 for hydrogen bond? Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: A) ch4 b) nah c) nh3 d) bh3 e) hi previous question next question It is an ionic bond, hydrogen is more electronegative than oxygen, oxygen occupies more space than hydrogen, oxygen is more electronegative than hydrogen, or it is a hydrogen bond Web which of the following molecules can form hydrogen bonds? There are exactly the right numbers of δ + hydrogens and lone pairs for every one of them to be involved in hydrogen bonding. Two with the hydrogen atoms and two with the with the oxygen atoms. Web can ch4 form hydrogen bonds.
Web can ch4 form hydrogen bonds. Hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule. Web which of the following molecules can form hydrogen bonds? No, hydrogen bonding only occurs where hydrogen is bonded to nitrogen (n), oxygen (o), and fluorine (f). Web so technically ch4 cannot hydrogen bond (certainly not between it's own molecules). View the full answer transcribed image text: This is because hydrogen bonds are a type of electrostatic interaction, which is only possible in molecules in which. It is an ionic bond, hydrogen is more electronegative than oxygen, oxygen occupies more space than hydrogen, oxygen is more electronegative than hydrogen, or it is a hydrogen bond No it can't form hydrogen bonds.electro negativity is not sufficient. There are exactly the right numbers of δ + hydrogens and lone pairs for every one of them to be involved in hydrogen bonding.
Which have a higher boiling point ch3nh2 or ch4 or sh2? No, hydrogen bonding only occurs where hydrogen is bonded to nitrogen (n), oxygen (o), and fluorine (f). Hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule. Which of the following molecules can form hydrogen bonds? This is because hydrogen bonds are a type of electrostatic interaction, which is only possible in molecules in which. No it can't form hydrogen bonds.electro negativity is not sufficient. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: Web can ch4 for hydrogen bond? Web can ch4 form hydrogen bonds? 4) which of the following molecules can form hydrogen bonds?
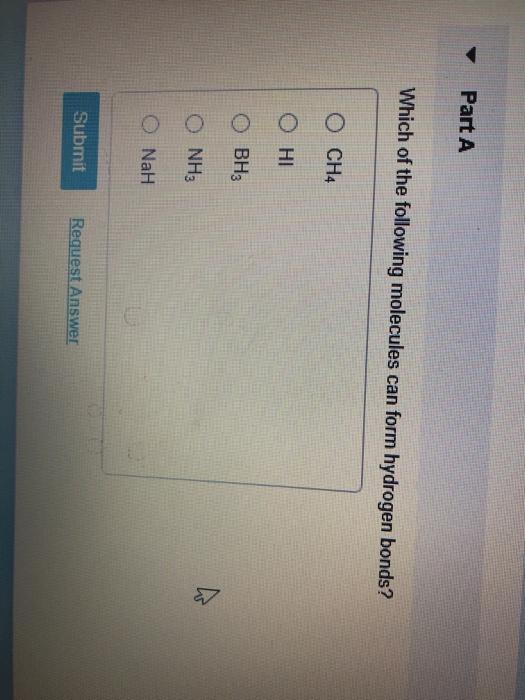
Part A Which of the following molecules can form hydrogen bonds? NaH
Ch4 cannot form hydrogen bonds. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web so technically ch4 cannot hydrogen bond (certainly not between it's own molecules). No, hydrogen bonding only occurs where hydrogen is bonded to nitrogen (n), oxygen (o), and fluorine (f). Two with the hydrogen atoms and two with.
Bonds That Hold Water Molecules Together / Intermolecular Forces
A) ch4 b) nah c) nh3 d) bh3 e) hi previous question next question Which have a higher boiling point ch3nh2 or ch4 or sh2? Web so technically ch4 cannot hydrogen bond (certainly not between it's own molecules). Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: This is because hydrogen bonds are.
Why Is Hydrogen Bonding Only Possible With Hydrogen
Web can ch4 for hydrogen bond? No, hydrogen bonding only occurs where hydrogen is bonded to nitrogen (n), oxygen (o), and fluorine (f). Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Web you'll get a detailed solution from a subject matter expert that helps you learn core.
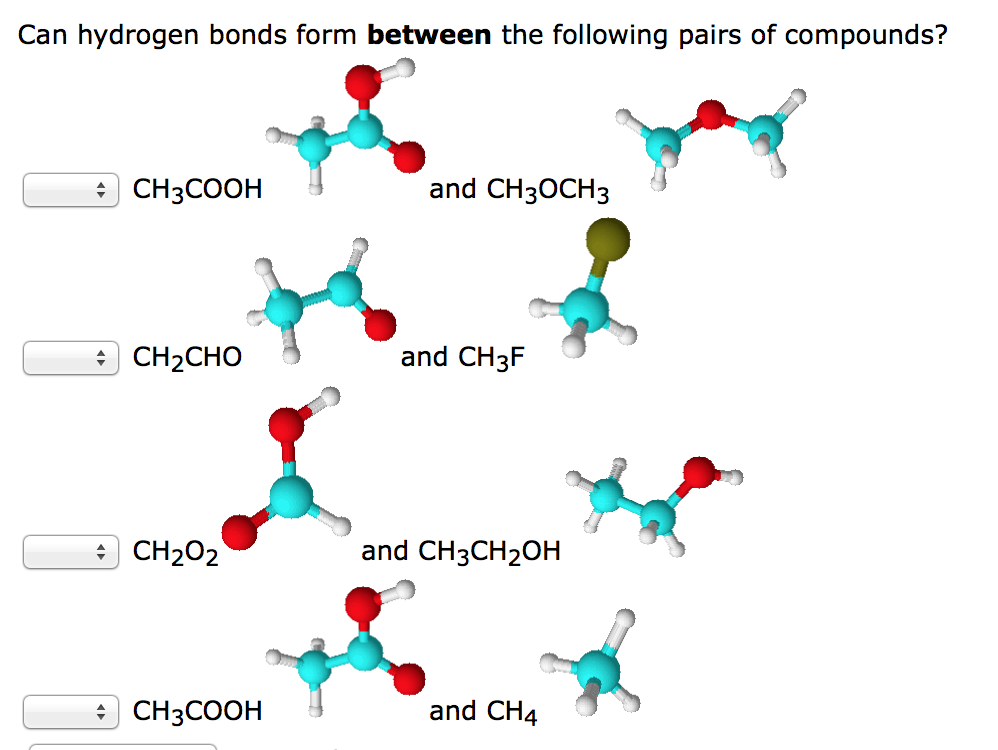
Solved Can hydrogen bonds form between the following pairs
Which of the following molecules can form hydrogen bonds? No it can't form hydrogen bonds.electro negativity is not sufficient. Web can ch4 for hydrogen bond? Web can ch4 form hydrogen bonds? Web which of the following molecules can form hydrogen bonds?
Solved Can hydrogen bonds form between the following pairs
Such a bond is weaker than an ionic bond or covalent bond but stronger than van der waals forces. A) ch4 b) nah c) nh3 d) bh3 e) hi previous question next question It is an ionic bond, hydrogen is more electronegative than oxygen, oxygen occupies more space than hydrogen, oxygen is more electronegative than hydrogen, or it is a.
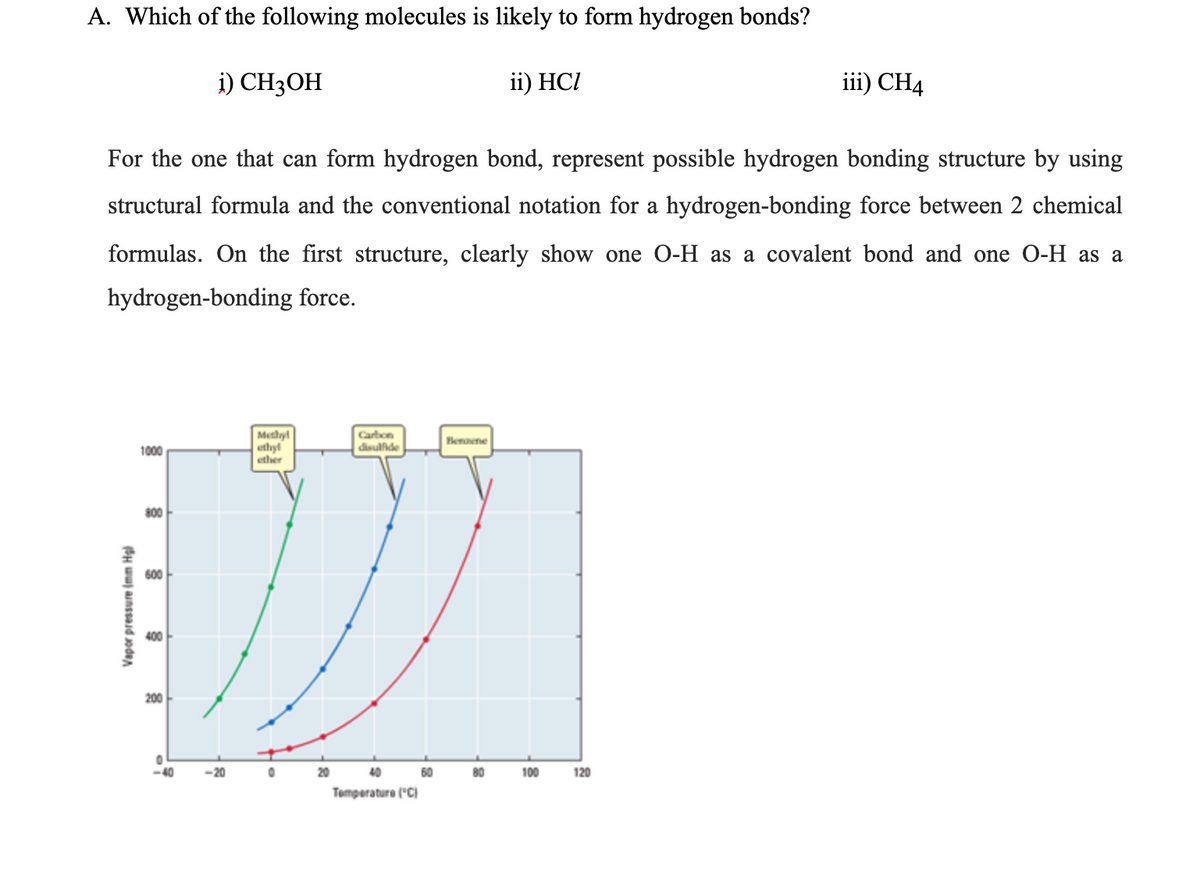
Answered A. Which of the following molecules is… bartleby
Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; No, hydrogen bonding only occurs where hydrogen is bonded to nitrogen (n), oxygen (o), and fluorine (f). Two with the hydrogen atoms and two with the with the oxygen atoms. 4) which of the following molecules can form hydrogen.
Solved Part A Which of the following molecules can form
Two with the hydrogen atoms and two with the with the oxygen atoms. Web can ch4 form hydrogen bonds. Web which of the following molecules can form hydrogen bonds? Web can ch4 form hydrogen bonds? No it can't form hydrogen bonds.electro negativity is not sufficient.
Can Ch4 Form Hydrogen Bonds CANZI
Web can ch4 form hydrogen bonds. Web which of the following molecules can form hydrogen bonds? 4) which of the following molecules can form hydrogen bonds? Which of the following molecules can form hydrogen bonds? Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Ch4 Polar Or Nonpolar Solved Of Molecular Valence Formula Electrons
Web can ch4 form hydrogen bonds. A) ch4 b) nah c) nh3 d) bh3 e) hi previous question next question No, hydrogen bonding only occurs where hydrogen is bonded to nitrogen (n), oxygen (o), and fluorine (f). There are exactly the right numbers of δ + hydrogens and lone pairs for every one of them to be involved in hydrogen.
Solved Which of the following molecules can form hydrogen
4) which of the following molecules can form hydrogen bonds? Web so technically ch4 cannot hydrogen bond (certainly not between it's own molecules). Web can ch4 form hydrogen bonds? No, hydrogen bonding only occurs where hydrogen is bonded to nitrogen (n), oxygen (o), and fluorine (f). View the full answer transcribed image text:
Such A Bond Is Weaker Than An Ionic Bond Or Covalent Bond But Stronger Than Van Der Waals Forces.
Web can ch4 form hydrogen bonds. View the full answer transcribed image text: Hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule. There are exactly the right numbers of δ + hydrogens and lone pairs for every one of them to be involved in hydrogen bonding.
No, Hydrogen Bonding Only Occurs Where Hydrogen Is Bonded To Nitrogen (N), Oxygen (O), And Fluorine (F).
Web can ch4 form hydrogen bonds? Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; This is because hydrogen bonds are a type of electrostatic interaction, which is only possible in molecules in which. Web which of the following molecules can form hydrogen bonds?
A) Ch4 B) Nah C) Nh3 D) Bh3 E) Hi Previous Question Next Question
Web so technically ch4 cannot hydrogen bond (certainly not between it's own molecules). Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Ch4 cannot form hydrogen bonds. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules:
Which Of The Following Molecules Can Form Hydrogen Bonds?
Web can ch4 for hydrogen bond? No it can't form hydrogen bonds.electro negativity is not sufficient. Two with the hydrogen atoms and two with the with the oxygen atoms. 4) which of the following molecules can form hydrogen bonds?