Ammonium Nitrite Reacts To Form Nitrogen And Water
Ammonium Nitrite Reacts To Form Nitrogen And Water - Web ted under certain conditions, the substance ammonium nitrite can be broken down to form nitrogen and water if 32.7 grams of ammonium nitrite react to form 14.3 grams of. Under certain conditions, the substance ammonium nitrite can be broken down to form nitrogen and water. Web as you can see, ammonium nitrite decomposes to form nitrogen gas, n_2, and water, h_2o, according to the unbalanced chemical equation. Write the balanced chemical equation that represents the chemical statement. Web ammonium ions and nitrite ions react in water to form nitrogen gas. Web interesting facts about ammonium nitrate. Web ammonium nitrate is a salt, thus, it easily dissolves in water. Ammonium nitrate is a chemical compound with the formula nh4no3. Web an alternate version of ammonium nitrite's chemical formula. Web this reaction mechanism releases the nitrite ion (no2−) to produce an intermediate (nitrosamine (h2nno)) which dissociates very rapidly to form the final.
Web as you can see, ammonium nitrite decomposes to form nitrogen gas, n_2, and water, h_2o, according to the unbalanced chemical equation. These are the most reduced forms of the inorganic. The unique thing about this experiment is the fact that water is actually not part of the chemical equation. Web from the reaction is given that the ammonium nitrate ‾ \underline{\text{ammonium nitrate}} ammonium nitrate (n h 4 n o 2) \mathrm{(nh_4no_2)} (n h 4 n o 2 ) reacts to form. Web ammonium nitrate is a salt, thus, it easily dissolves in water. Web nitrate and ammonium nitrogen are measured for all surface and ground water samples. This problem has been solved! If 27.6 grams of ammonium nitrite react to. Web this reaction mechanism releases the nitrite ion (no2−) to produce an intermediate (nitrosamine (h2nno)) which dissociates very rapidly to form the final. The commercial grade contains about 33.5 percent.
Under certain conditions, the substance ammonium nitrite can be broken down to form nitrogen and water. The decomposition reaction is nh4no3. Web ted under certain conditions, the substance ammonium nitrite can be broken down to form nitrogen and water if 32.7 grams of ammonium nitrite react to form 14.3 grams of. Write the balanced chemical equation that represents the chemical statement. Web (c) sodium hydrogen carbonate reacts to form sodium carbonate, water, and carbon dioxide. Nh → n + 2h. These are the most reduced forms of the inorganic. Web interesting facts about ammonium nitrate. The unique thing about this experiment is the fact that water is actually not part of the chemical equation. This problem has been solved!
(a) Profiles of influent ammonium, nitrite nitrogen concentrations
It is composed of nitric acid and salt of ammonia. (d) ammonium nitrite reacts to form nitrogen and water. Web ammonium nitrate, (nh 4 no 3 ), a salt of ammonia and nitric acid, used widely in fertilizers and explosives. At room temperature, it decomposes into water and nitrogen. Web from the reaction is given that the ammonium nitrate ‾.
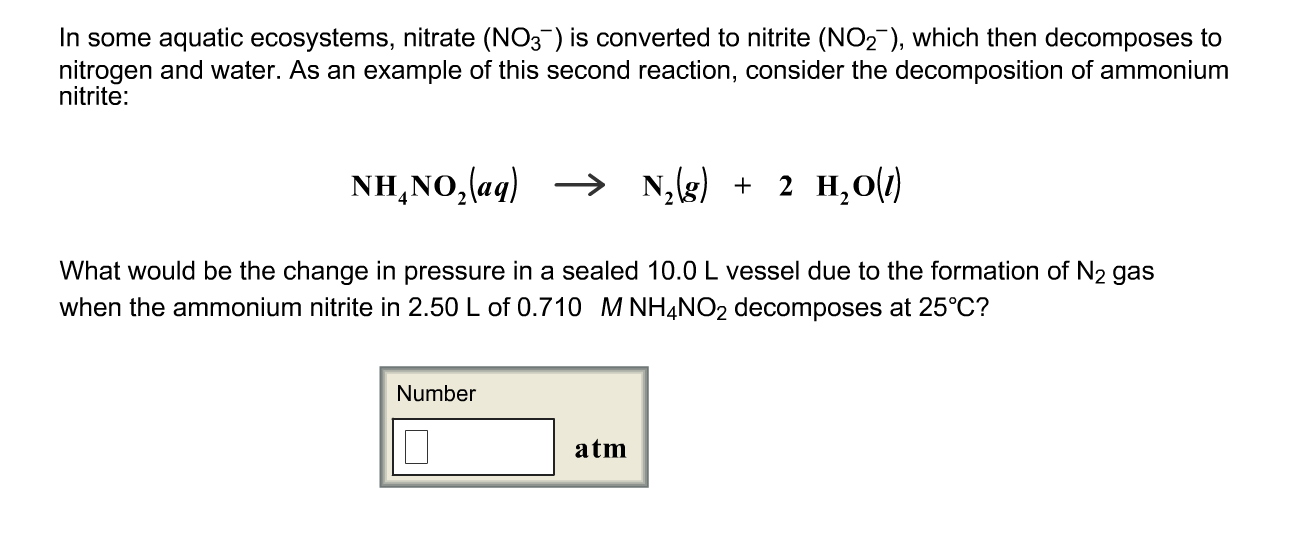
Solved In some aquatic ecosystems, nitrate (NO_3^) is
Ammonium nitrate decomposes under heat into n2o and h2o. Under certain conditions, the substance ammonium nitrite can be broken down to form nitrogen and water. Web ammonium nitrate, (nh 4 no 3 ), a salt of ammonia and nitric acid, used widely in fertilizers and explosives. Web an alternate version of ammonium nitrite's chemical formula. It is composed of nitric.
(a) Profiles of influent ammonium, nitrite nitrogen concentrations
Web ammonium ions and nitrite ions react in water to form nitrogen gas. If 27.6 grams of ammonium nitrite react to. Web interesting facts about ammonium nitrate. Web ammonium nitrate, (nh 4 no 3 ), a salt of ammonia and nitric acid, used widely in fertilizers and explosives. Web ammonia nitrogen is reductive, and the concentration of ammonia nitrogen can.
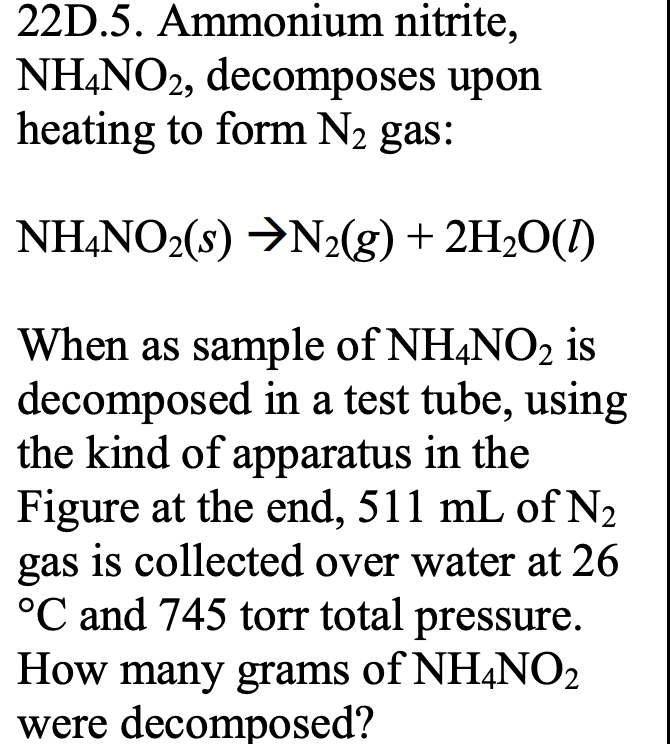
Solved 22D.5. Ammonium nitrite, NH4NO2, upon
Ammonium nitrate decomposes under heat into n2o and h2o. Web if 21.1 grams of ammonium nitrite react to form 9.2 grams of nitrogen, how many grams of water must simultaneously be formed? The commercial grade contains about 33.5 percent. Web interesting facts about ammonium nitrate. Write the balanced chemical equation that represents the chemical statement.
The nitrogen cycle. The main pool of N in the soil is in the form of
At room temperature, it decomposes into water and nitrogen. Web ammonium nitrate is a salt, thus, it easily dissolves in water. Under certain conditions, the substance ammonium nitrite can be broken down to form nitrogen and water. The commercial grade contains about 33.5 percent. Web if 21.1 grams of ammonium nitrite react to form 9.2 grams of nitrogen, how many.
Nitrite field test kit Asianmedic
Under certain conditions, the substance ammonium nitrite can be broken down to form nitrogen and water. The unique thing about this experiment is the fact that water is actually not part of the chemical equation. Write the balanced chemical equation that represents the chemical statement. The commercial grade contains about 33.5 percent. If 27.6 grams of ammonium nitrite react to.
Solved provide complete answers. 1. An aqueous solution of
Web ammonium ions and nitrite ions react in water to form nitrogen gas. Nh → n + 2h. At room temperature, it decomposes into water and nitrogen. Ammonium nitrate is a chemical compound with the formula nh4no3. If 27.6 grams of ammonium nitrite react to.
Ammonium, nitrite, and nitrate nitrogen concentrations at the end of
Web if 21.1 grams of ammonium nitrite react to form 9.2 grams of nitrogen, how many grams of water must simultaneously be formed? If 27.6 grams of ammonium nitrite react to. Web nitrate and ammonium nitrogen are measured for all surface and ground water samples. This problem has been solved! Web (c) sodium hydrogen carbonate reacts to form sodium carbonate,.
Ammonium Nitrite, Organic And Solvents Triveni Chemicals in
Web as you can see, ammonium nitrite decomposes to form nitrogen gas, n_2, and water, h_2o, according to the unbalanced chemical equation. This problem has been solved! Web nitrate and ammonium nitrogen are measured for all surface and ground water samples. Web ammonium ions and nitrite ions react in water to form nitrogen gas. Web ammonia nitrogen is reductive, and.
Ammonium Nitrite Facts, Formula, Properties, Uses
Web ammonia nitrogen is reductive, and the concentration of ammonia nitrogen can be detected indirectly by converting ammonia nitrogen into nitrate or nitrite. The commercial grade contains about 33.5 percent. Web an alternate version of ammonium nitrite's chemical formula. Web as you can see, ammonium nitrite decomposes to form nitrogen gas, n_2, and water, h_2o, according to the unbalanced chemical.
If 27.6 Grams Of Ammonium Nitrite React To.
At room temperature, it decomposes into water and nitrogen. Web (c) sodium hydrogen carbonate reacts to form sodium carbonate, water, and carbon dioxide. Ammonium nitrate decomposes under heat into n2o and h2o. It is composed of nitric acid and salt of ammonia.
Web As You Can See, Ammonium Nitrite Decomposes To Form Nitrogen Gas, N_2, And Water, H_2O, According To The Unbalanced Chemical Equation.
The decomposition reaction is nh4no3. Web this reaction mechanism releases the nitrite ion (no2−) to produce an intermediate (nitrosamine (h2nno)) which dissociates very rapidly to form the final. Web ammonium nitrate is a salt, thus, it easily dissolves in water. Web from the reaction is given that the ammonium nitrate ‾ \underline{\text{ammonium nitrate}} ammonium nitrate (n h 4 n o 2) \mathrm{(nh_4no_2)} (n h 4 n o 2 ) reacts to form.
These Are The Most Reduced Forms Of The Inorganic.
Web an alternate version of ammonium nitrite's chemical formula. Ammonium nitrate is a chemical compound with the formula nh4no3. Nh → n + 2h. Web ammonium nitrate decomposes into nitrogen and water?
Web Interesting Facts About Ammonium Nitrate.
Write the balanced chemical equation that represents the chemical statement. Web ted under certain conditions, the substance ammonium nitrite can be broken down to form nitrogen and water if 32.7 grams of ammonium nitrite react to form 14.3 grams of. Under certain conditions, the substance ammonium nitrite can be broken down to form nitrogen and water. Web ammonium ions and nitrite ions react in water to form nitrogen gas.