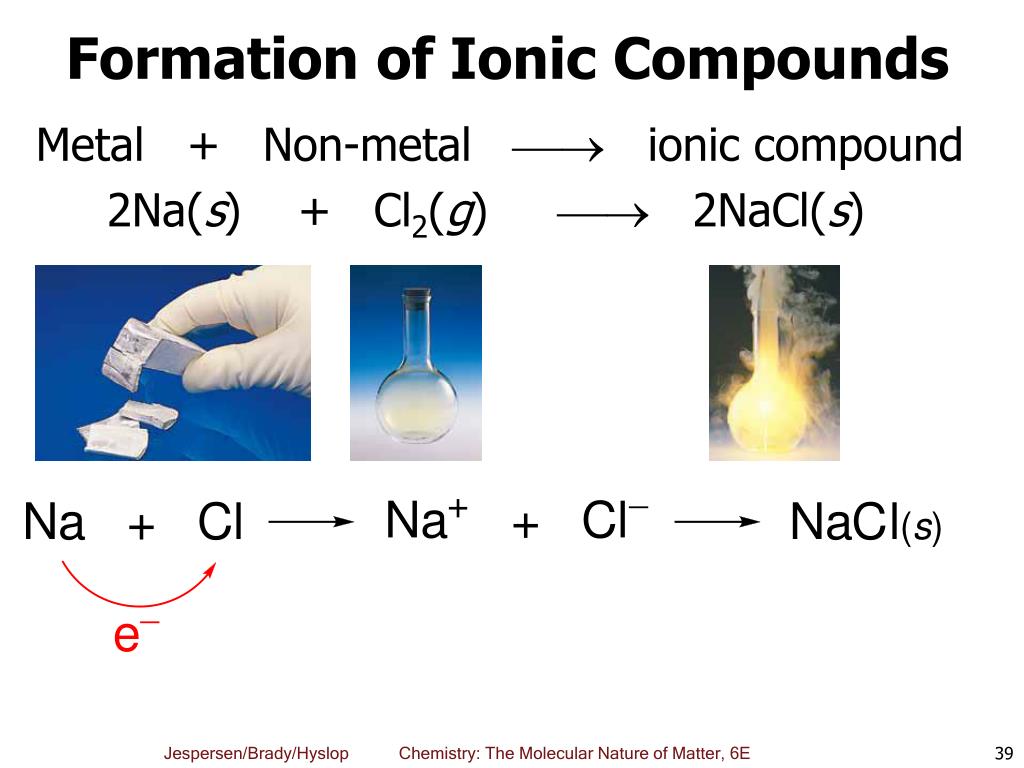
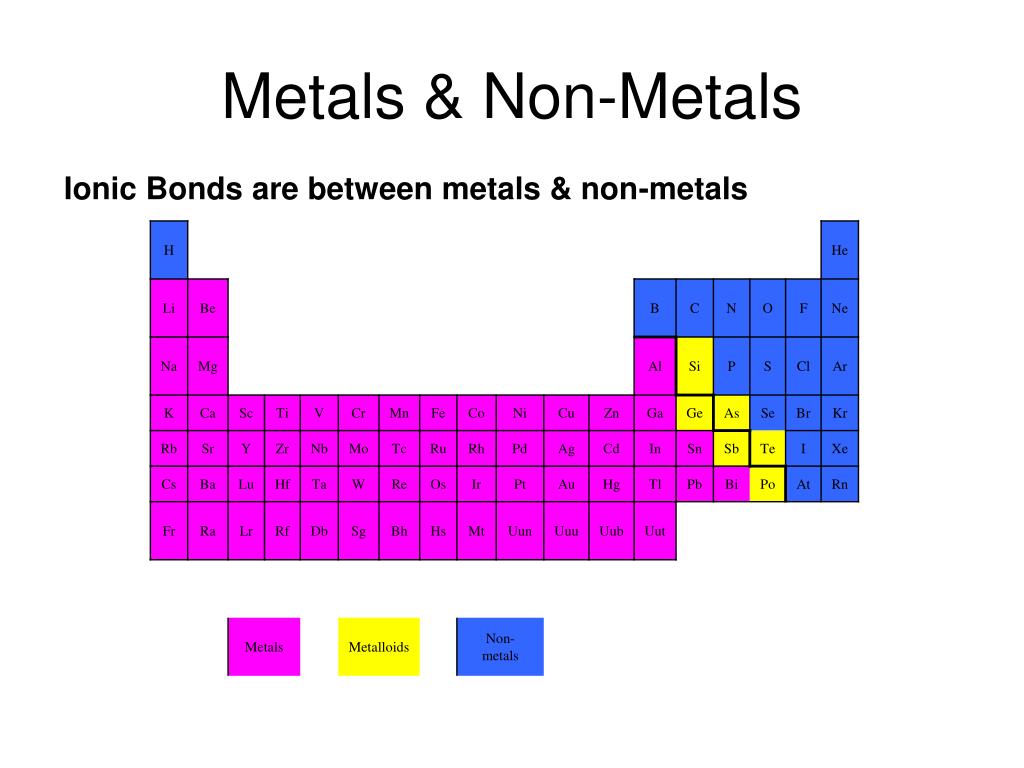
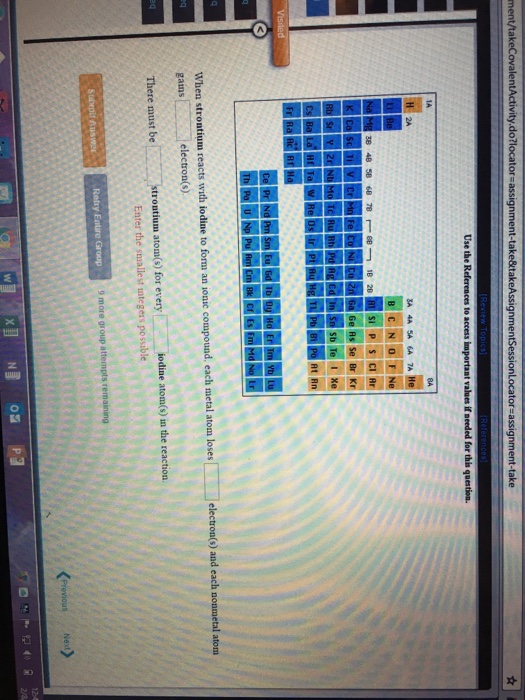
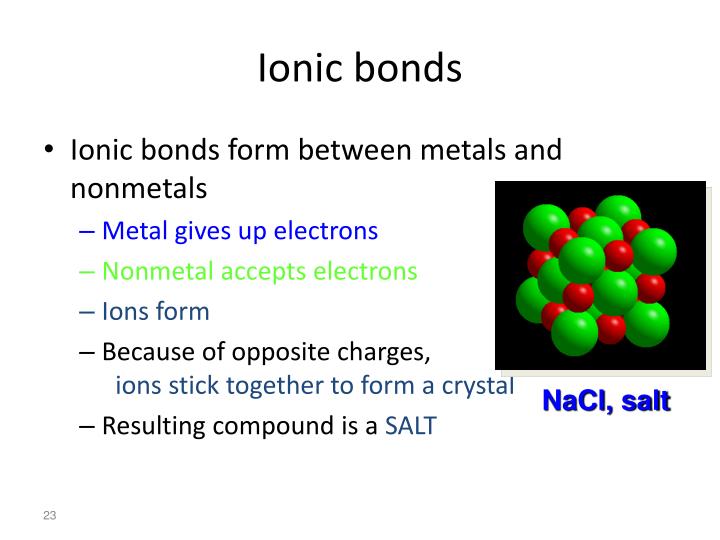
A Metal And A Nonmetal Form An Ionic Compound
A Metal And A Nonmetal Form An Ionic Compound - A metal and a nonmetal form an. On the other hand, compounds formed between two or. When metals react with nonmetals, their atoms become charged. The formula of an ionic compound. Web the statement is false. The ionization energy of an element describes the amount of energy needed to cause an atom to lose an electron. Web ionic compounds are compounds formed between a metal and nonmetal which have a crystalline lattice structure. They can conduct electricity and are usually highly water. An ionic bond is an electrostatic attraction between the positive and negative ions of a chemical compound. Web identify the type of reaction for each of the following as combination, decomposition, single replacement, double replacement, or combustion:
The formula of an ionic compound. To obtain a full outer shell: They differ in their structure and properties. Web identify the type of reaction for each of the following as combination, decomposition, single replacement, double replacement, or combustion: A metal and a nonmetal form an. The ionization energy of an element describes the amount of energy needed to cause an atom to lose an electron. When metals react with nonmetals, their atoms become charged. The periodic table can help us recognize many of the compounds that are. Web the metals form cations, the nonmetals form anions, and the resulting compounds are solids under normal conditions. Covalent bonds consist of pairs of electrons.
Web a compound that contains ions and is held together by ionic bonds is called an ionic compound. Web an ionic compound has a giant lattice structure. They can conduct electricity and are usually highly water. The formula of an ionic compound. The bond formed between them is. A metal and a nonmetal form an. Web the name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion (the name. Web ions form when atoms lose or gain electrons. On the other hand, compounds formed between two or. Web identify the type of reaction for each of the following as combination, decomposition, single replacement, double replacement, or combustion:
Nonmetal Elements Science notes, Periodic table, Periodic table of
Metals tend to have low. Web the statement is false. Web ionic compounds are compounds formed between a metal and nonmetal which have a crystalline lattice structure. The bond formed between them is. The periodic table can help us recognize many of the compounds that are.
PPT Chapter 3 Elements, Compounds, and the Periodic Table PowerPoint
Web ionic compounds are compounds formed between a metal and nonmetal which have a crystalline lattice structure. They differ in their structure and properties. Covalent bonds consist of pairs of electrons. Web the metals form cations, the nonmetals form anions, and the resulting compounds are solids under normal conditions. Web ions form when atoms lose or gain electrons.
PPT Ionic Compounds Formula to Name PowerPoint Presentation, free
They differ in their structure and properties. Web an ionic compound has a giant lattice structure. Web ions form when atoms lose or gain electrons. When metals react with nonmetals, their atoms become charged. To obtain a full outer shell:
Solved When Strontium Reacts With Iodine To Form An Ionic...
Web an ionic compound has a giant lattice structure. Web the metals form cations, the nonmetals form anions, and the resulting compounds are solids under normal conditions. The bond formed between them is. The periodic table can help us recognize many of the compounds that are. The formula of an ionic compound.
PPT Noble Gases and Valence e Ionization Energy and Bonding
Web ionic compounds are compounds formed between a metal and nonmetal which have a crystalline lattice structure. The formula of an ionic compound. Web ions form when atoms lose or gain electrons. Web identify the type of reaction for each of the following as combination, decomposition, single replacement, double replacement, or combustion: The periodic table can help us recognize many.
The Parts of the Periodic Table
On the other hand, compounds formed between two or. Web the name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion (the name. A metal and a nonmetal form an. The bond formed between them is. The ionization energy of an element describes.
Ions Predict Charge Stone Cold Chemistry Talk Ions Predict Charge
The formula of an ionic compound. They differ in their structure and properties. Web identify the type of reaction for each of the following as combination, decomposition, single replacement, double replacement, or combustion: Web the metals form cations, the nonmetals form anions, and the resulting compounds are solids under normal conditions. A metal and a nonmetal form an.
Ionic Bond Definition Easy Hard Science
To obtain a full outer shell: The bond formed between them is. The formula of an ionic compound. An ionic bond is an electrostatic attraction between the positive and negative ions of a chemical compound. Web ionic compounds are compounds formed between a metal and nonmetal which have a crystalline lattice structure.
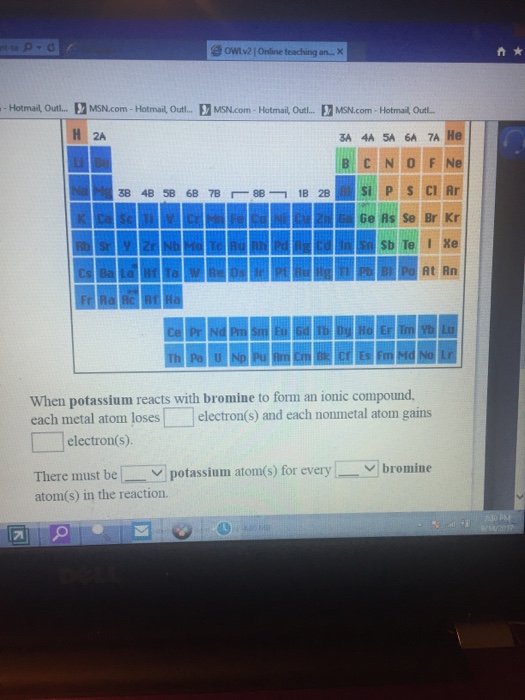
Solved When potassium reacts with bromine to form an ionic
Web a compound that contains ions and is held together by ionic bonds is called an ionic compound. Covalent bonds consist of pairs of electrons. The bond formed between them is. An ionic bond is an electrostatic attraction between the positive and negative ions of a chemical compound. When metals react with nonmetals, their atoms become charged.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Web a compound that contains ions and is held together by ionic bonds is called an ionic compound. To obtain a full outer shell: Web ions form when atoms lose or gain electrons. The formula of an ionic compound. When metals react with nonmetals, their atoms become charged.
They Can Conduct Electricity And Are Usually Highly Water.
Metals tend to have low. The periodic table can help us recognize many of the compounds that are. The formula of an ionic compound. Web ionic compounds are compounds formed between a metal and nonmetal which have a crystalline lattice structure.
Web An Ionic Compound Has A Giant Lattice Structure.
A metal and a nonmetal form an. An ionic bond is an electrostatic attraction between the positive and negative ions of a chemical compound. Web a compound that contains ions and is held together by ionic bonds is called an ionic compound. To obtain a full outer shell:
When Metals React With Nonmetals, Their Atoms Become Charged.
Web identify the type of reaction for each of the following as combination, decomposition, single replacement, double replacement, or combustion: They differ in their structure and properties. The ionization energy of an element describes the amount of energy needed to cause an atom to lose an electron. Web ions form when atoms lose or gain electrons.
On The Other Hand, Compounds Formed Between Two Or.
Web the statement is false. Covalent bonds consist of pairs of electrons. The bond formed between them is. Web the metals form cations, the nonmetals form anions, and the resulting compounds are solids under normal conditions.