Iso 14971:2019 Risk Management Plan Template
Iso 14971:2019 Risk Management Plan Template - Oliver eidel template download this is a free template,. Web templates iso 14971 templates updated june 20, 2023 template: Web risk management plan template , which can be used as starting point for the practical implementation of the risk management process; Risk management activities shall be planned. Web the purpose of this procedure is to describe the risk management process in accordance with iso 14971. Iso 14971:2019 has been recognized as the consensus standard by. Click here to get this previously confidential risk management plan template in accordance with the requirements of iso 14971:2019 or. Web the risk management plan. This document could be used as guidance in developing and maintaining a risk management. Web established principles of risk management that have evolved over many years.
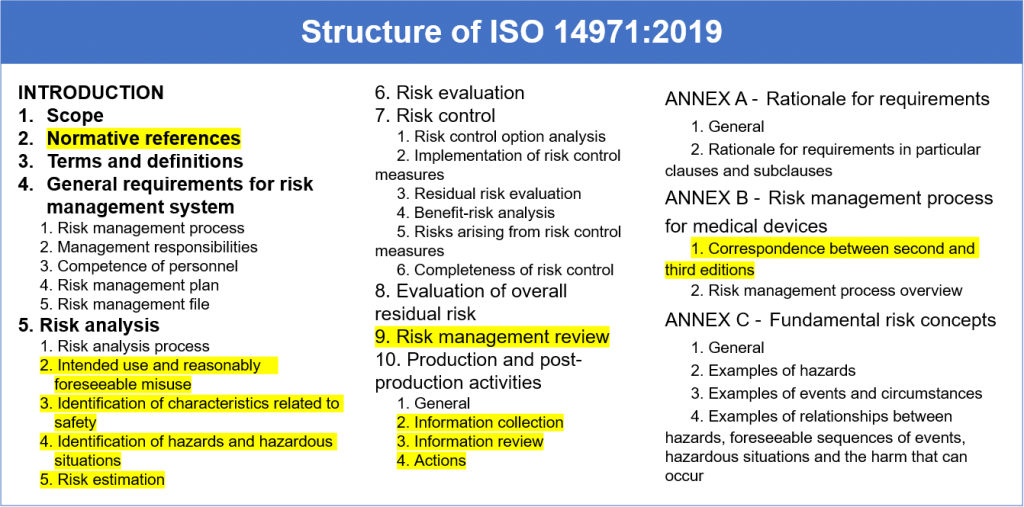
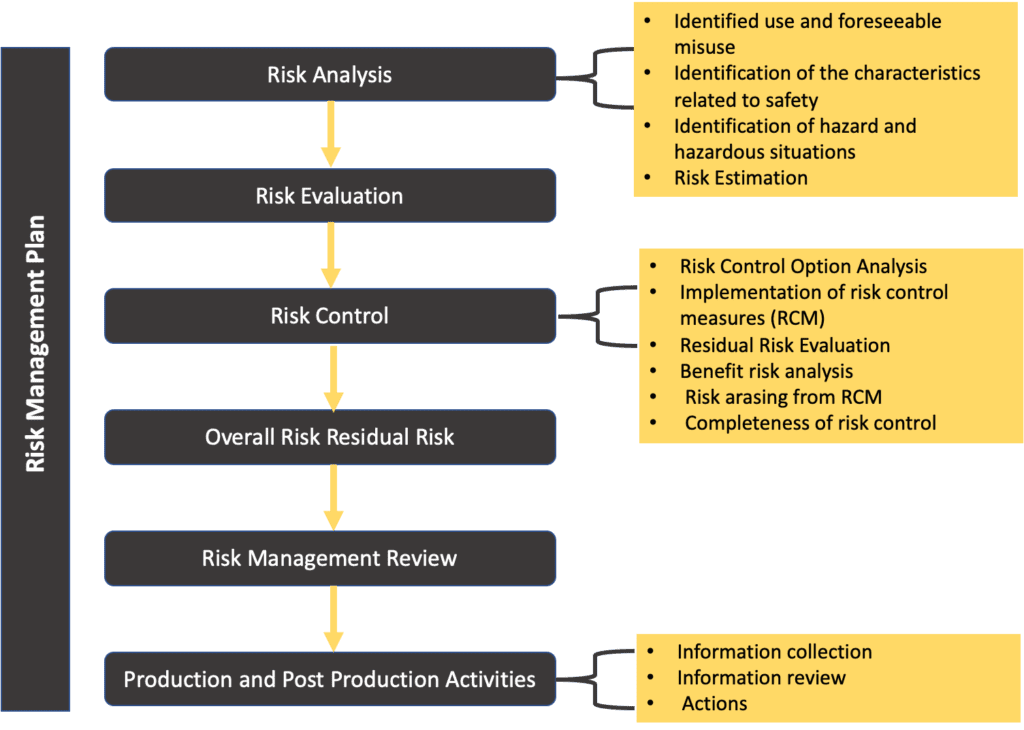
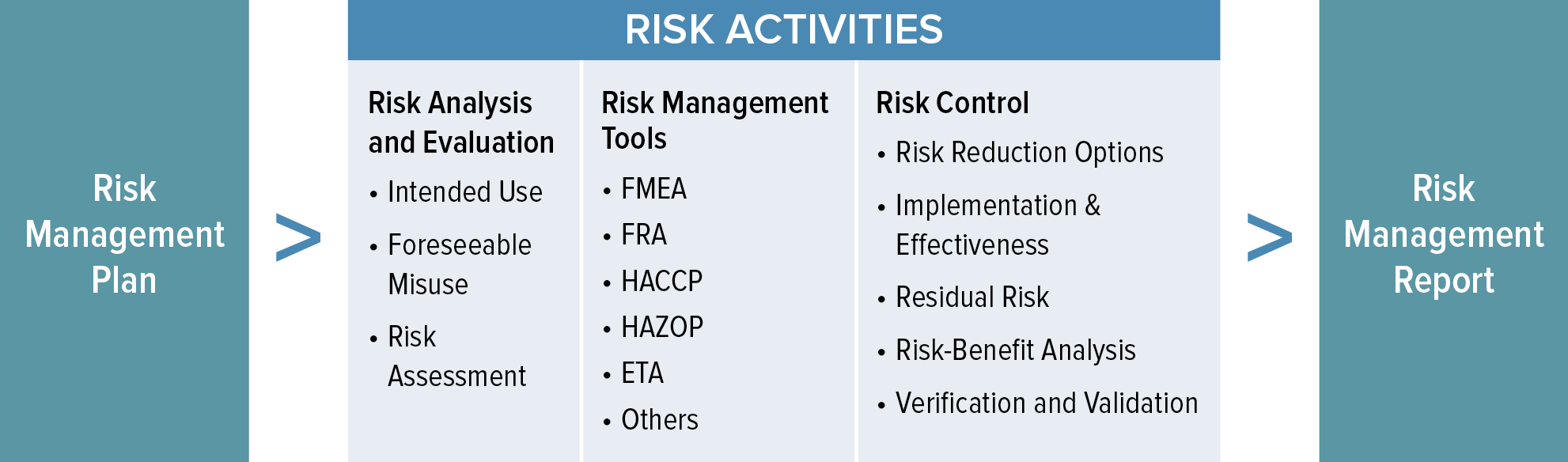
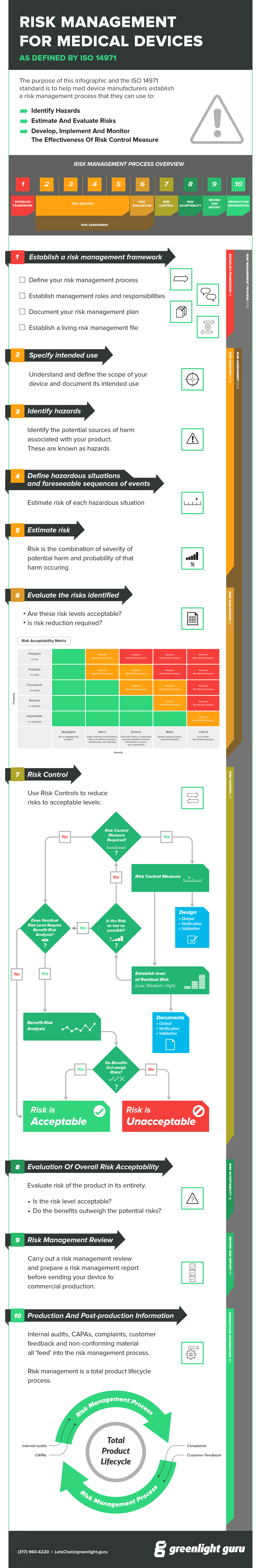
Web the risk management process described in the new iso 14971 consists of several steps: Web iso 14971:2019 is the international standard for risk management in medical devices. Web established principles of risk management that have evolved over many years. This document could be used as guidance in developing and maintaining a risk management. Web the risk management plan. Risk management activities shall be planned. Web the purpose of this procedure is to describe the risk management process in accordance with iso 14971. Web templates iso 14971 templates updated june 20, 2023 template: A new requirement to establish a method to evaluate the overall residual risk and criteria for. Web risk management plan template , which can be used as starting point for the practical implementation of the risk management process;
Oliver eidel template download this is a free template,. Web iso 14971:2019 medical devices — application of risk management to medical devices abstract preview this document specifies terminology, principles and a process for risk. Web the risk management process described in the new iso 14971 consists of several steps: Web the risk management plan. The focus of this blog post is the first of these six steps: Web in iso 14971:2019, section 4.4, the standard states that: Web what's new in iso 14971:2019 key definitions implementing iso 14971 initiating risk management and design controls part 1: Scope of responsibilities and the individual phases. Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and evaluate the risks. Click here to get this previously confidential risk management plan template in accordance with the requirements of iso 14971:2019 or.
What is new in ISO 149712019 Medical Device HQ
The focus of this blog post is the first of these six steps: Click here to get this previously confidential risk management plan template in accordance with the requirements of iso 14971:2019 or. Iso 14971:2019 has been recognized as the consensus standard by. Oliver eidel template download this is a free template,. A new requirement to establish a method to.
ISO 149712019 Changes in the Current Version of ISO 14971 Oriel
Web risk management plan template , which can be used as starting point for the practical implementation of the risk management process; Oliver eidel template download this is a free template,. The focus of this blog post is the first of these six steps: This document could be used as guidance in developing and maintaining a risk management. A new.
Iso14971 Risk Management Template / Risk Management Procedure
The focus of this blog post is the first of these six steps: A new requirement to establish a method to evaluate the overall residual risk and criteria for. Iso 14971:2019 has been recognized as the consensus standard by. Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical.
Medical Device Risk Management Updates What is New in ISO 149712019?
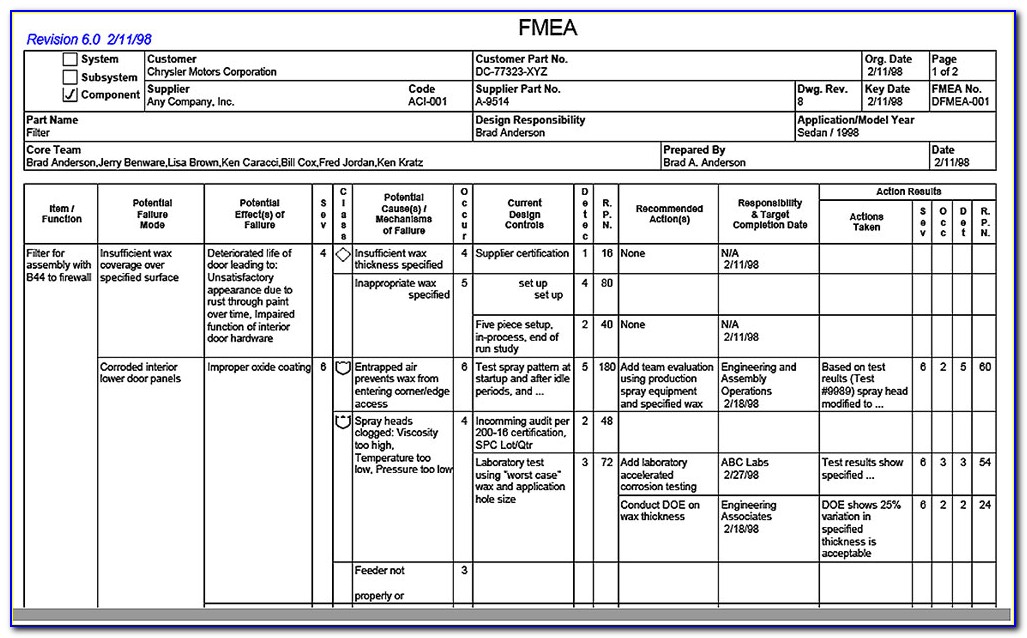
Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and evaluate the risks. A new requirement to establish a method to evaluate the overall residual risk and criteria for. Scope of responsibilities and the individual phases. Risk management activities shall be planned. Web the purpose of.
ISO 149712019 Update for Risk Management Process
For the particular medical device being considered, the. Web the risk management process described in the new iso 14971 consists of several steps: Web iso 14971:2019 medical devices — application of risk management to medical devices abstract preview this document specifies terminology, principles and a process for risk. Web the purpose of this procedure is to describe the risk management.
Iso 14971 Risk Management Plan Example
A new requirement to establish a method to evaluate the overall residual risk and criteria for. Oliver eidel template download this is a free template,. Web the risk management process described in the new iso 14971 consists of several steps: Web risk management plan template , which can be used as starting point for the practical implementation of the risk.
ISO 14971 2019 Information & Training Medical DevicesPresentationEZE
Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and evaluate the risks. Web iso 14971:2019 medical devices — application of risk management to medical devices abstract preview this document specifies terminology, principles and a process for risk. This document could be used as guidance in.
ISO 149712019 Basics of Medical Device Risk Management
Web what's new in iso 14971:2019 key definitions implementing iso 14971 initiating risk management and design controls part 1: This document could be used as guidance in developing and maintaining a risk management. Web iso 14971:2019 is the international standard for risk management in medical devices. Web the risk management plan. Web iso 14971 specifies a process through which the.
ISO 14971 Risk Management for Medical Devices The Definitive Guide
Web established principles of risk management that have evolved over many years. Web what's new in iso 14971:2019 key definitions implementing iso 14971 initiating risk management and design controls part 1: A new requirement to establish a method to evaluate the overall residual risk and criteria for. Web templates iso 14971 templates updated june 20, 2023 template: Web the method.
Iso14971 Risk Management Template Third edition of ISO 14971
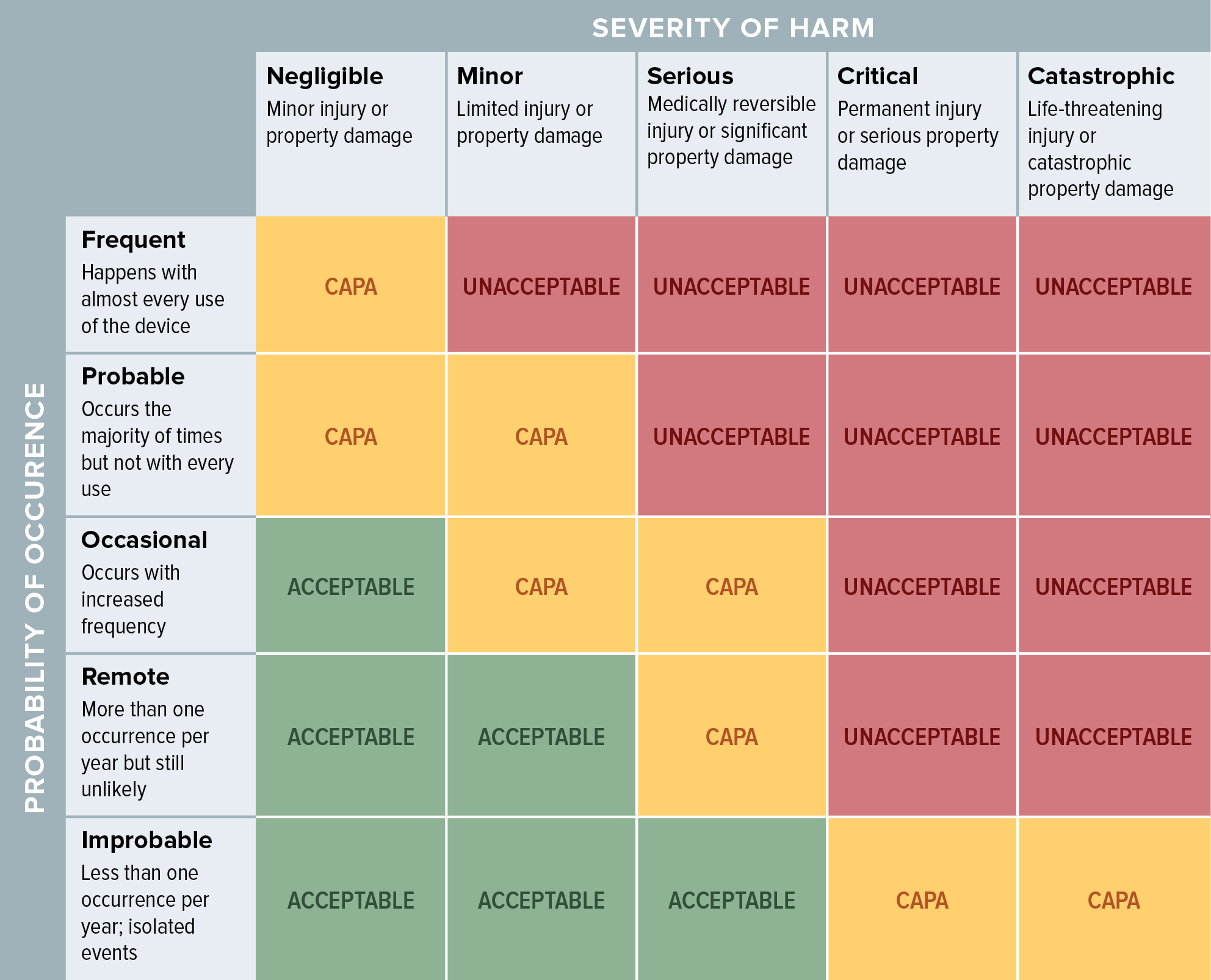
Web the risk management process described in the new iso 14971 consists of several steps: Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and evaluate the risks. Web the method for the evaluation of the overall residual risk and the criteria for its acceptability are.
The Focus Of This Blog Post Is The First Of These Six Steps:
A new requirement to establish a method to evaluate the overall residual risk and criteria for. Web iso 14971:2019 is the international standard for risk management in medical devices. Web what's new in iso 14971:2019 key definitions implementing iso 14971 initiating risk management and design controls part 1: Web risk management plan template , which can be used as starting point for the practical implementation of the risk management process;
Web The Purpose Of This Procedure Is To Describe The Risk Management Process In Accordance With Iso 14971.
Click here to get this previously confidential risk management plan template in accordance with the requirements of iso 14971:2019 or. Risk management activities shall be planned. Iso 14971:2019 has been recognized as the consensus standard by. Web iso 14971:2019 medical devices — application of risk management to medical devices abstract preview this document specifies terminology, principles and a process for risk.
Web The Risk Management Process Described In The New Iso 14971 Consists Of Several Steps:
Web templates iso 14971 templates updated june 20, 2023 template: Web the risk management plan. This document could be used as guidance in developing and maintaining a risk management. Web recently, the third version of iso 14971:2019 series has been notified and several aspects of this regulation include the best objectives to be achieved by the.
Web An Iso 14971 Checklist Is A Form Based On The Guidelines Of Iso 14971, A Voluntary International Standard That Details How To Apply Risk Management Practices For.
Web established principles of risk management that have evolved over many years. Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and evaluate the risks. Web the method for the evaluation of the overall residual risk and the criteria for its acceptability are required to be defined in the risk management plan. Scope of responsibilities and the individual phases.